Acetic Acid
Acetic Acid is a synthetic carboxylic acid. It is a monocarboxylic acid with two carbon atoms. It is a clear, colorless organic liquid that has a pungent odor like vinegar. is a solution of acetic acid and water that contains 5%-20% acetic acid by volume. An undiluted solution of acetic acid is known as glacial acetic acid. It forms ice-like crystals when the temperature falls below 16 degrees Celsius.
The IUPAC name of acetic acid is ethanoic acid. Its formal name is also ethanoic acid, but it is usually referred to as acetic acid, which is derived from the Latin word acetum, which means vinegar.
Acetic Acid Formula I Structure of Acetic Acid
In the solid-state, in the chain of molecules, the molecules are connected to each other by hydrogen bonds. Its chemical formula is CH3COOH or its structure is given by CH3(C=O) OH, or CH3CO2H.
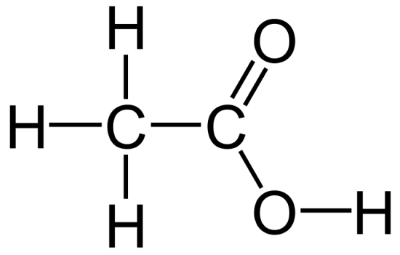
It is a carboxylic acid that contains a methyl group (CH3) connected to a carboxyl group. We can also say that the acetyl group (CH3CO) is connected to the hydroxyl group (OH). It has sp2 hybridization and occurs as a dimer in a liquid or vapor state due to intermolecular hydrogen bonding.
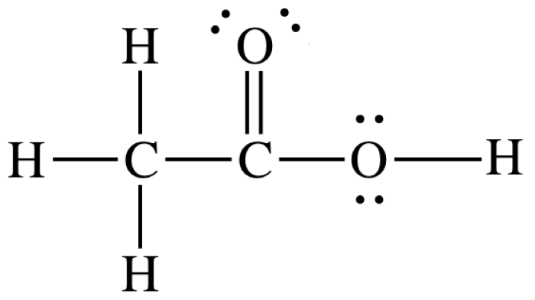
Lewis structure of Acetic Acid
It is clear from the below Lewis structure of acetic acid that it has six single bonds and one double bond. Further, each oxygen atom contains two lone pairs (total four electrons) of electrons. Each dash shows one bond in which two electrons are bonded to each other.
Physical Properties of Acetic Acid
- It is a colorless liquid with a pungent smell.
- Its melting point is 289K and its boiling point is 391K at STP.
- Its molar mass is 60.052 g/mol and its density in the liquid form is 1.049 g.cm.-3
- It is a polar, protic solvent in liquid form with a dielectric constant of 6.2.
- It has a corrosive nature. So, it can cause blisters when comes in contact with the skin.
Chemical Properties of Acetic Acid
- The carboxyl functional group in acetic acid can cause ionization of the compound, such as CH3COOH ? CH3COO- + H+
- The release of the proton due to ionization of acetic acid is the reason for the acidic quality of acetic acid.
- Its acid dissociation constant (pKa) in a solution of water is 4.76.
- Its conjugate base is acetate (CH3COO-).
- The pH of its solution of 1.0M concentration is 2.4, so it does not dissociate completely.
Acetic Acid Reactions
Acetic acid can undergo all the chemical reactions of other carboxylic acids as described below:
-
Decomposition
At a temperature of more than 440 degrees Celsius, it undergoes decomposition to produce either methane and carbon or water and ethenone as shown below:
CH3COOH + heat → CO2 + CH4
CH3COOH + heat → H2C=C=O + H2O
-
Reaction with metals
Few metals such as magnesium, zinc, and iron undergo corrosion when exposed to acetic acid. Acetate salts are formed in these reactions. For example, when ethanoic acid reacts with magnesium, magnesium acetate is formed with the release of hydrogen gas as shown below:
2CH3COOH + Mg → Mg (CH3COO)2 (magnesium acetate) + H2
-
Reaction with alkalis
Acetic acid when reacts with alkalis forms acetate salts as shown below:
CH3COOH + KOH → CH3COOK + H2O
-
Reaction with carbonates
Acetic acid forms acetate salts when reacts with carbonates. Carbon dioxide and water are also formed.
2CH3COOH + Na2CO3 (sodium carbonate) → 2CH3COONa + CO2 + H2O
CH3COOH + NaHCO3 (sodium bicarbonate) → CH3COONa + CO2 + H2O
Uses of Acetic Acid
Acetic acid has numerous uses. Some of the major uses of acetic acid are as follows:
- It is used in the manufacturing of dyes, plastics, silk, perfumes, and more.
- It is also used as a table vinegar.
- It is used as a local irritant in medicines.
- It is used as a coagulation agent in the rubber industry.
- It is required in the formation of acetone, acetate, esters, etc.
- It is important for the production of VAM (vinyl acetate monomer).
- It is also used in the treatment of cancer.
- It is an important industrial solvent because of its desirable solvent properties and its ability to form miscible mixtures with both polar and nonpolar compounds. So, it is widely used to prepare dimethyl terephthalate (DMT) on a large scale or in industries.
- It is also used as a micro bacterial disinfectant.
Glacial Acetic Acid
Glacial acetic acid refers to an acetic acid that contains very less water (less than 1%). It is also known as anhydrous (water-free) acetic acid. It is called glacial as it tends to solidify at low temperatures less than 16 degrees Celsius.
It can be formed by dripping acetic acid solution over a frozen "stalactite" of acetic acid. The pure acid sticks to the glacial acetic acid, whereas, the impurities flow with the liquid. Acetic acid is a weak acid; considered safe to drink in vinegar. However, glacial acetic acid is corrosive in nature, so it can harm the skin on contact.
Preparation of Acetic Acid
Acetic acid is produced through the carbonylation of methanol. The three steps involved in this method as described below:
- CH3OH (methanol) + HI (hydrogen iodide) →CH3l (methyl iodide intermediate) + H2O
- CH3I + CO (carbon monoxide) →CH3Col (acetyl iodide)
- CH3COI + H2O → CH3COOH (acetic acid) + HI
In the first step, a methyl iodide intermediate is formed when methanol reacts with hydrogen iodide. In the second step, the intermediate (methyl iodide) reacts with carbon monoxide to form acetyl iodide. In the third step, the acetyl iodide is reacted with water that leads to the formation of acetic acid along with the hydrogen iodide.
Other Methods of Acetic Acid Preparation
By oxidation:
-
The oxidation of acetaldehyde by using some naphthalene salts of chromium, cobalt, manganese, etc., results in the formation of acetic acid.
O2 + 2CH3CHO → 2CH3COOH
-
The oxidation of ethylene (C2H4) by using palladium catalyst and a heteropoly acid also results in the formation of acetic acid, as shown below:
O2 + C2H4 → CH3COOH
Furthermore, some anaerobic bacteria can also convert sugar into acetic acid directly through anaerobic fermentation without involving the formation of ethanol intermediate, as shown below:
C6H12O6 → 3CH3COOH
Interesting Facts about Acetic Acid
- It is the second simplest carboxylic acid, after formic acid.
- It is mainly used in vinegar, and in the formation of cellulose acetate and polyvinyl acetate.
- It is used as a food additive for flavor and to maintain acidity.
- Around 6 metric tons of acetic acid are used worldwide in a year.
- Most of the acetic acid is produced by using petrochemical feedstock.
- It forms acetate when ionizes at physiological pH.
- Acetic acid bacteria like Acetobacter and Clostridium acetobutlicum also produce acetic acid.
- It is also produced by fruits when they ripen.
- The acetyl group when joined to coenzyme A, acts as a holoenzyme that is used in the metabolism of fat and carbohydrates.
|


 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










