AcetoneAcetone is an organic compound. Its chemical formula is C3H6O and is also known as propane. It is a highly flammable colorless and volatile substance. Acetone is commonly found in the exhaust released from vehicles, plants, forest fires, etc. In the human body, it is present in urine and blood. It is produced through ketosis in the human body. Besides this, it is also found in volcanic gases. History of AcetoneAcetone was first discovered by alchemists in the Middle Ages, during that time it was known as 'spirit of Saturn'. Its chemical formula was given by French chemist Jean Baptiste Dumas and German chemist Justus von Liebig. The name Acetone is given by a French chemist in 1833 by adding a suffix to acetic acid. By 1852, acetone was said to be methyl acetate by an English chemist Alexander William Williamson. During World War 1, Chaim Weizmann introduced a process for the industrial production of acetone. This process was called Weizmann Process. Acetone Structure and FormulaThe structural or organic formula of acetone with its structure is given below. Its structure helps understand acetone and its organic formula better. 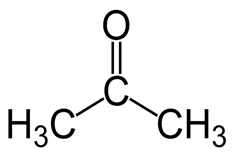
The molecule of acetone is made of 3 carbon, 6 hydrogens and one oxygen atom. It is a ketone as it contains a carbonyl group present in it. So, the chemical formula of the acetone is CH3COCH3 and the condensed formula is C3H6O. Its IUPAC name is 2-propanone. It is also called dimethyl ketone. Besides this, it is the simplest ketone made of a three-carbon chain in which two of the hydrogen atoms in the second carbon are substituted by an oxygen atom bonded to a carbon atom by double bonds. Physical Properties of Acetone
Chemical properties of Acetone
Furthermore, it has a carbonyl group (C=O) at the center, which causes polarization as carbon is less electronegative than oxygen. So, it is used as a reactant with nucleophilic molecules that are attracted toward the carbonyl carbon lacking in electrons. Acetone ProductionThe United States is the largest producer of acetone. It produces around 1.70 million tonnes per year. After the United States, acetone is also produced on a large scale in Taiwan and China. Traditional MethodIn the early days or the traditional method of acetone production involved dry distillation of acetates compounds like calcium acetate. See the chemical reaction below; Ca (CH3COO) 2 → CaO (s) + CO2 (g) + (CH3)2 CO (v) However, during World War 1, acetone was produced by fermentation of acetone butanol ethanol with bacteria Clostridium acetobutylicum, a method introduced by Chaim Weizmann. This method was abandoned as newer methods were introduced that were more productive. Current MethodMost of the acetone, nearly 80%, is produced by the cumene process in the industry. In this process, the benzene is alkylated with propylene to produce cumene. This cumene is made to undergo oxidation in the presence of air and a small amount of radical initiator (as a reagent) to produce phenol and acetone. Benzene is a stable compound so it does not react easily. So, a radical initiator is used as a catalyst to activate the benzene ring for the reaction. The atmospheric oxygen plays the role of an oxidizing agent. It is a cheap method as it uses less costly raw materials: benzene and propylene. So it is widely used for the production of acetone. 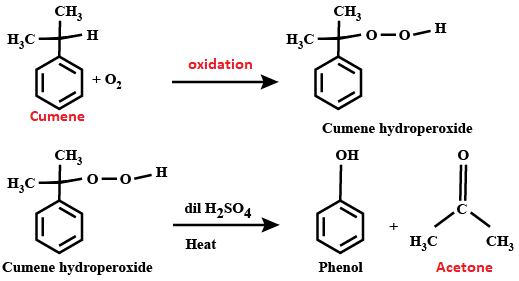
Besides this, in Wacker Hoechst process, oxidation or hydration of propylene compound takes place that forms 2-propanol, which is later oxidized to form acetone. Laboratory preparation of Acetonei) Dry distillation of Calcium Acetate It can be prepared by the dry distillation of calcium acetate as shown below; (CH3COOH)2 Ca → CH3COCH3 + CaCO3 ii) Dehydration of 2-propanol It can also be prepared by dehydration of alcohol. Due to dehydration, alcohol forms the carbonyl group on the carbon atom which has an alcohol group on it. For example, see the dehydration of 2-propanol below; CH3CH(OH)CH3 → CH3COCH3 iii) By oxidation of 2-propanol CH3CH(OH)CH3 → CH3COCH3 iv) By reductive oxidation of 2-propene CH3CH=CH2 + O3 → CH3COCH3 Acetone UsesAcetone has innumerable uses. It can be used in different ways as described below: Solvent: It is widely used as a solvent for many plastics and synthetic fibers. Nearly one-third of the total acetone produced in the world is used as a solvent and the remaining is consumed as acetone cyanohydrin. Chemical synthesis: Acetone is used in the synthesis of methyl methacrylate. It is also used in the formation of bisphenol A, methyl isobutyl alcohol and methyl isobutyl ketone solvents. Medical and cosmetic use: Acetone is used for medical and cosmetic purposes and as a compound in food additives. It is used to thin most cosmetic products such as face creams, moisturizers, lotions, etc. It is used with alcohol for the treatment of acne and is also used to clean or sterilize medical tools and equipment. Laboratory: In the laboratories, it is used as a polar and aprotic solvent in many organic reactions such as jones oxidation reactions. It is also used to clean labs glassware as it is cheap and volatile in nature. Pharmaceuticals: In the pharma industry, acetone is widely used as a solvent in the preparation of medicines. Electronics: It is used as a solvent to clean electronic devices, gadgets, appliances, etc. Other uses
Acetone is a highly flammable solvent so the container containing acetone should be properly closed and it should not be close to stoves, fire or heat-producing sources. If you are going to store a large amount of acetone, store it in a fire-proof container. Precautions while using or working with AcetoneAcetone can be harmful if not handled carefully. By taking the following precautions you can use it without harming yourself as well as others.
Acetone Boiling PointEach substance has a unique boiling point. An unknown substance can be identified by determining its boiling point and then comparing it with reference values. The boiling point of a substance is the temperature at which it starts transforming into vapor and its vapor pressure is equal to the atmospheric pressure or the pressure exerted by the surroundings on the liquid. The boiling point of acetone is 56 degrees Celsius. Acetone is a polar molecule. So, dipole-dipole interactions occur between acetone molecules in the liquid phase. These interactions are not as strong as the hydrogen bonds between water molecules. So, less energy is required to change the liquid phase of acetone to vapor phase, and thus it has a low boiling point than water. The boiling point of a substance depends on the pressure of its surroundings. For example, in the mountainous regions, the atmospheric pressure is low so, the water boils at a temperature below 100 degrees Celsius. This is because the vapor pressure becomes equal to the atmospheric pressure before the temperate reaches 100 degrees Celsius.
Next TopicSalicylic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










