Amino Acids DefinitionIntroductionOrganic substances known as amino acids have both amino & carboxylic acid functional groups. Even though there are many hundreds of amino acids in nature, alpha-amino acids, which constitute protein, are by far the most important ones. There are 22 alpha amino acids present in a genetic code. 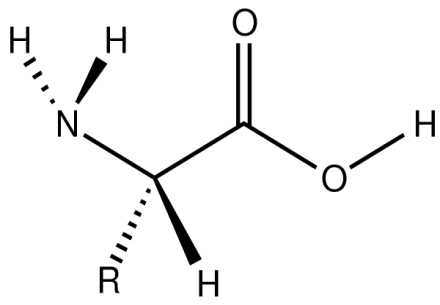
Alpha-, beta-, gamma-, or delta-amino acids were divided according to their structure and function and further classified according to polarity, ionization, and the kind of side chain group. After water, proteins made of amino acid residues make up the second-largest portion of human muscle and tissue. Besides serving as protein residues, amino acids are also taken involvement in the transfer of neurotransmitters and biosynthesis. They are believed to have been a major factor in the origin of life on our planet. The IUPAC-IUBMB Joint Committee on Biological Nomenclature officially names amino acids using the fake "neutrality" structure shown in the figure. For instance, alanine is known scientifically as 2-amino propanoic acid derived from the formula CH3CH(NH2)COOH. Here is how the Commission defended this strategy: The comprehensive names and formulae are for hypothetical forms with unprotonated amino groups and undissociated carboxyl groups. This approach helps avoid several nomenclatural issues but should not be interpreted as meaning that these structures account for a significant portion of amino acid molecules. History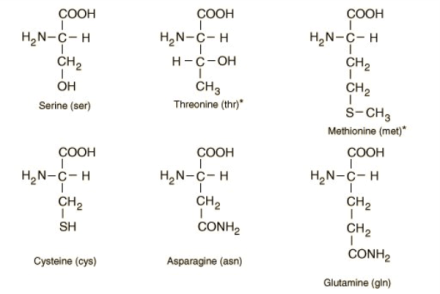
In the early 1800s, the initial few amino acids were identified. Asparagine, the first amino to be found, was extracted from asparagus in 1806 by French scientists Louis-Nicolas Vauquelin & Pierre Jean Robiquet. Cystine was identified in 1810, but cysteine, its monomer, was not until 1884. Around 1820, glycine and leucine were found. William Cumming Rose, who not only identified the necessary amino acids and defined the minimal daily needs of all amino acids for healthy development, made the last discovery of the 20 known amino acids in 1935: threonine. Wurtz acknowledged the uniformity of the molecular category in 1865, although he did not give it a specific name. The phrase "amino acid" was first used in English in 1898; the German name, Aminosäure, was first used earlier. During enzyme action or acid hydrolysis, amino acids have been identified to come from proteins. Emil Fischer & Franz Hofmeister separately postulated in 1902 that proteins comprise a large number of amino acids and that these interactions between one amino acid's amino group and another's carboxyl group result in a linear arrangement that Fischer dubbed "peptides." Physiochemical PropertiesThe Twenty canonical amino acids may be divided into groups based on their behavior. Charge, hydrophilicity, hydrophobicity, size, & functional groups are significant variables. These characteristics influence protein structure and interactions of protein. Leu, Ile, Val, Phe, and Trp are hydrophobic residues that are often buried in the center of water-soluble proteins, while hydrophilic side chains were exposed to the solvent. (Remember that a residue in biochemistry refers to a particular monomer inside the polymer molecules of a polysaccharide, protein, or nucleic acid.) The outer ring of accessible hydrophobic amino acids on the membrane proteins usually serves as anchors for the proteins in the lipid bilayer. A band of hydrophobic amino acids found on the surface of several peripheral membrane proteins causes them to adhere to the membrane. Like charged particle molecules having surfaces rich in negative charge amino acids like glutamate and aspartate, negative charge molecules have surfaces rich in charged amino acids such as lysine and arginine, and vice versa. For instance, the low-complexity sections of the nucleic-acid binding domain include significant quantities of the amino acids lysine and arginine. The scales of hydrophobicity for amino acid residues vary. Some acids have unique qualities. Glycine is more elastic than other amino acids, and proline creates a cycle to the polypeptide backbone. Other cysteine residues and cysteine may bind covalently via disulfide bonds. Whereas cysteine, phenylalanine, tryptophan, methionine, valine, leucine, and isoleucine are the opposite and are highly reactive, complex, or hydrophobic, glycine and proline are significantly abundant in low complexity sections of both eukaryotic and prokaryotic proteins. Numerous proteins go through various posttranslational modifications. For instance, when a signaling protein has cysteine residues that could have the fatty acid palmitic acid introduced to it and then removed, it can connect to and then separate from a cell membrane. Different chemical groups are sometimes attached to the side chains of amino acid residues to produce hydrophobic lipoproteins or glycoproteins, which are hydrophilic, enabling the protein to bind to a membrane momentarily. General Framework
The 21 eukaryotic proteinogenic amino acids are categorized based on the charges carried by their side chains at physiological pH. (7.4) R denotes a side chain unique to each amino acid in the structure at the top of the page. The -carbon is the carbon atom adjacent to the carboxyl group. The term "amino acid" refers to amino acids with an amino group immediately linked to the -carbon. They include the secondary amines proline and hydroxyproline[b]. These were formerly often referred to as amino acids, which is incorrect since they lack the imine grouping HN=C. IsomerismThe most prevalent natural forms of amino acids are called -amino acids because they include the structural components NH + 3 (or NH + 2 in the case of proline) and CO 2 functional groups connected to the same C atom. Natural amino acids are the only ones with the L configuration and are exclusively present in proteins during translation in the ribosome, except for achiral glycine. Instead of the amino acid's optical activity, the L and D convention for amino acid configuration relates to the optical activity of the isomer of glyceraldehyde from which the amino acid may be produced D-glyceraldehyde is dextrorotatory and L-glyceraldehyde is levorotatory. The (S) and (R) designators may be used as an alternate convention to describe the absolute configuration. Proteins include almost all (S) amino acids, with the exceptions of cysteine (R) and glycine (non-chiral). Cysteine's side chain is geometrically situated in the same location as the side chains of the remaining amino acids. Still, the Cahn-Ingold-Prelog sequence rules prioritize the side chain over the carboxyl oxygen, resulting in the R/S nomenclature being inverted. This contrasts with other side chains, which have a lower priority than the carboxyl group due to their atom composition. Occasionally, proteins include D-amino acid residues created as a posttranslational modification from l-amino acids. Side ChainsWhen the amino nitrogen atom is joined to the -carbon, the carbon atom next to the carboxylate group, amino acids Occurrence and FunctionAs they help make proteins, amino acids with the amine group linked to the carbon adjacent to the carboxyl are particularly relevant to living things. These are often referred to as 2-, alpha-, or -amino acids (generic formula: H2NCHRCOOH, where R is a chemical substituent termed as a "side chain"); frequently, the phrase "amino acid" is used to refer to them explicitly. They consist of the 22 proteinogenic (protein-building) amino acids, which are combined to create peptide chains (or "polypeptides"), the fundamental units of a wide variety of proteins. All of these are L-stereoisomers, or "left-handed" enantiomers, except a few D-amino acids, or "right-handed" enantiomers, which may be found in certain antibiotics, bacterial envelopes, and D-serine, a neuromodulator. Many amino acids, both proteinogenic and non-proteinogenic, have biological use. For instance, glutamate or gamma-aminobutyric acid nonstandard gamma-amino acid are the main inhibitory & excitatory neurotransmitters in the human brain. Proline is converted into hydroxyproline, a crucial part of the collagen in connective tissue. Red blood cell-useable porphyrins are biosynthesized from glycine. Using carnitine helps move lipids. Since the human body cannot synthesize nine proteinogenic amino acid residues from other substances, they must be consumed via food. These amino acids are referred to be "essential" for humans. At particular ages or medical circumstances, others can be deemed conditionally necessary. Species-to-species variations in essential amino acids are also possible. Amino acids are crucial for nutrition and are often utilized in dietary supplements, fertilizers, feeds, and food technology due to their biological importance. Industrial applications include the creation of chiral catalysts, biodegradable polymers, and pharmaceuticals. 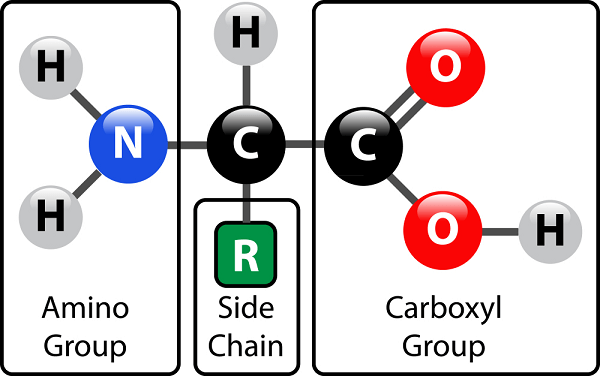
Peptide-Producing Amino AcidsProteins are built from amino acids. They combine via condensation processes to create either shorter polymer chains termed peptides or proteins, or longer chains known as polypeptides. Each amino acid residue in these chains is connected to two nearby amino acids, resulting in linear, unbranched chains. An mRNA template, an RNA copy of one of the organism's genes, reads the genetic code and determines the sequence in which the amino acids are added. The process of creating proteins in nature that DNA/RNA encodes is known as translation, and it entails a ribozyme called a ribosome adding amino acids one at a time to a building protein chain. Proteinogenic are the 22 amino acids that are naturally integrated into polypeptides. Twenty of them are represented by the whole genetic code. Selenocysteine and pyrrolysine, the remaining 2, are integrated into proteins using certain synthetic methods. Certain methanogenic archaea employ pyrrolysine in the enzymes they use to generate methane. Whenever the mRNA being converted has a SECIS element, the UGA codon instead of encoding a stop codon, selenocysteine is inserted. It is represented by the codon UAG, which is often a stop codon in some species. Just after this UAG codon, a PYLIS backward sequence follows. Gly, Ala, Asp, Val, Ser, Pro, Glu, Leu, and Thr may be among a group of amino acids that made up the earliest genetic code, so although Cys, Met, Tyr, Trp, His, and Phe may be among a group of amino acids that made up later additions to the genetic code, according to several independent evolutionary studies. Normative vs. Non-Normative Amino AcidsStandard or conventional amino acids refer to the 20 amino acids directly represented by codons of the human genetic code. In bacteria, mitochondria, and chloroplasts, methionine is often replaced by a modified version of the amino acid called N-formylmethionine as the first amino acid of proteins. Such amino acids are referred to as non-canonical or nonstandard. Most nonstandard amino acids also are non-proteinogenic, which means that they cannot be changed into proteins throughout the translation. Nevertheless, two of these nonstandard amino acids can be converted into proteins during translation using data not included in the global genetic code. Selenocysteine is present in most eukaryotes and non-eukaryotes but is not directly coded by DNA, and pyrrolysine is the two nonstandard proteinogenic amino acids. These unconventional amino acids are seldom incorporated. For instance, selenocysteine is included in the fundamental structures of 25 human proteins and is used as the catalytic moiety in the active regions of structurally defined enzymes (selenoenzymes). Via alternative codons, pyrrolysine & selenocysteine are encoded. For instance, stop codons and the SECIS element encodes selenocysteine. Instead of being treated as a distinct proteinogenic amino acid, N-formylmethionine is often the first amino acid of protein in bacteria, mitochondria, & chloroplasts. The genetic code may also be "expanded" by using non-natural codon-tRNA pairings to create new proteins called all proteins that include non-proteinogenic amino acids. Amino Acids that are not ProteinogenicAmino acids are not involved in protein synthesis. Several non-proteinogenic amino acids have been identified besides the 22 proteinogenic amino acids. They either are not synthesized directly and in isolation by typical cellular machinery (including levothyroxine, GABA, and carnitine), do not exist in proteins, or both. Posttranslational modification or modification carried out after translational during protein synthesis is how non-proteinogenic organic molecules present in proteins are created. These alterations are often necessary for a protein's regulation or function. For instance, glutamate's carboxylation improves the binding of calcium cations, and proline's hydroxylation produces hydroxyproline, which is present in collage. Another instance is the hypusine produced by altering a lysine residue in the translation initiation protein EIF5A. These alterations may also affect the protein's location; for instance, the insertion of lengthy hydrophobic groups can make a protein attach to just a phospholipid membrane. Certain amino acids are absent from proteins, however. Gamma-aminobutyric acid and 2-aminoisobutyric acid are two examples of this. Non-proteinogenic amino acids often act as mediators in the metabolic processes for other amino acids. For instance, ornithine and citrulline exist in the urea cycle, a step in the catabolism of amino acids. The amino acid beta-alanine, employed by microorganisms and plants in manufacturing pantothenic acid, acetyl - CoA A component, is a rare exception rather than the rule that -amino acids predominate throughout biology. Non-Protein FunctionThe neurotransmitter serotonin's precursor is tryptophan. Non-protein amino acids have significant functions in the human body as metabolic intermediaries, such as manufacturing the GABA neurotransmitters. The synthesis of several additional compounds involves the utilization of amino acids, including: Dopamine, epinephrine, norepinephrine, other catecholamine neurotransmitters, and several trace amines are precursors of tyrosine (and its precursor, phenylalanine). In people, phenylalanine serves as a precursor to tyrosine and phenethylamine. It serves as a plant precursor to many phenylpropanoids crucial to plant metabolism.
In Human-NutritionThe 20 standard amino acids that humans ingest from their diets are either used to make proteins and other macromolecules or as an energy source by being oxidized to urea & carbon dioxide. A transaminase removes the amino group to initiate the oxidation pathway, after which the amino group is supplied into the urea cycle. The other transamidation byproduct is an acid known as a keto acid that enters the citric acid cycle. By gluconeogenesis, glucose may also be produced from glucogenic amino acids. Nine of the 20 standard amino acids-His, Ile, Leu, Lys, Met, Phe, Thr, Trp, and Val-are referred to be necessary amino acids since the human body cannot produce them at the amount required for proper development from other chemicals, necessitating food consumption. In addition, children's taurine is a semi-essential amino sulfonic acid, and cysteine, tyrosine, & arginine are all regarded as semi-essential amino acids. The metabolic processes that create these monomers have not yet reached their full potential. It is challenging to generalize the dietary requirements for particular amino acids since the quantities needed also vary mostly on the age and health of the person. Human neurodegenerative disorders, including ALS, have been related to dietary exposure to a nonstandard amino acid BMAA. Industrial UsesFertilizerIn fertilizers, amino acids' capacity to chelate is occasionally utilized to speed up the supply of minerals into plants to address mineral shortages such as iron chlorosis. These fertilizers are also utilized to enhance the health status of the plants and stop deficiencies from arising. Animal chowBecause some ingredients in animal feed, like soybeans, have low levels of essential amino acids, particularly lysine, methionine, threonine, and tryptophan, amino acids are occasionally added to animal feed. Amino acids are also used to chelate metals to enhance the absorption of mineral deposits from feed supplements. FoodAmino acids are frequently added to food by manufacturers to help alleviate the symptoms of potassium deficiency, such as anemia, by improving mineral absorption and minimizing negative impacts from main inorganic supplementation. Particularly glutamic acid, which is used as a flavor enhancer, and aspartame, which is used as an artificial sweetener, are major consumers of amino acids in the food industry. Cosmetics and pharmaceuticalsSimilarly to this, the pharmaceutical sector uses several compounds of amino acids. These include eflornithine, a medication that suppresses ornithine decarboxylase and treats sleeping sickness, L-DOPA (L-dihydroxyphenylalanine), an experimental therapy for depression, and 5-HTP (5-hydroxytryptophan). Several cosmetics are created using amino acids. Expansion of the genetic codeSince 2001, 40 non-natural amino acids have been added to proteins using a special codon and a corresponding transfer RNA: aminoacyl - tRNA-synthetase pair to encode it with various physicochemical and biological properties. This allows for the creation of novel or improved proteins and the exploration of protein structure and function. NullomersWhile nullomers are recorders that theoretically code for such an amino acid, nature is biased against utilizing them in favor of other codons. For instance, bacteria prefer to use the codon CGA rather than AGA to code for arginine. As a result, certain sequences that do not exist in the genome are produced. This property may be exploited and utilized to develop novel, selective cancer treatments and to stop Genetic material from crime scenes from being contaminated. Chemical ComponentAs inexpensive feedstocks, amino acids are crucial. These substances serve as enantiomerically pure construction blocks in chiral pool synthesis. Even though there are no practical uses, amino acids have been studied as chiral catalyst precursors, particularly for asymmetric hydrogenation processes. Recyclable PlasticsThe building blocks of biodegradable materials, which may be used to create prosthetic implants and ecologically friendly packaging, have been thought to include amino acids. Polyaspartate is a liquid biodegradable polymer that has potential uses in agriculture and disposable diapers as an intriguing example of such materials. In addition to being a corrosion inhibitor and biodegradable anti-scaling agent, poly aspartate is a solubility and chelating agent for metal ions. Tyrosine, an aromatic amino acid, has also been considered a viable replacement for phenols like bisphenol A in producing polycarbonates.
Next TopicVelocity Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










