Atom DefinitionThe fundamental building blocks of everything, atoms determine the physical and chemical characteristics of all substances. Every object around us, from the air we breathe to the food we eat, is made up of atoms. These minuscule building blocks are composed of protons, neutrons, and electrons, among other even smaller particles. Protons and neutrons are found in the nucleus, while electrons orbit around the nucleus. Fundamental physics and chemistry laws regulate how these particles behave, they also enable researchers to comprehend and control the properties of matter. In this article, we will learn about the structure and properties of atoms, their role in the natural world, and their impact on modern technology. What Are AtomsAtoms are fundamental units of matter which make up everything in the universe. They can also be called as building blocks of the universe. They are incredibly small, so small that you cannot an atom with your naked eye. They are made up of three types of particles: protons, neutrons, and electrons. The nucleus of atoms contains protons and neutrons, and electrons orbit the nucleus in shells or energy levels. The number of protons in an atom's nucleus determines what element it is, and the arrangement of electrons around the nucleus determines the chemical properties of the element. Atoms can combine to form molecules, which are the basic units of compounds. Chemistry is the study of atoms and how they behave, and it helps us to understand how matter interacts and transforms in the environment. Structure of AtomsAtoms are made of even tinier particles inside them. As mentioned above, these sub-atomic particles are protons, neutrons, and electrons. Protons and neutrons reside in the nucleus of an atom, which is located at the center of the atom. Protons have a positive charge, while neutrons have no charge. Together, they make up the majority of the mass of the atom. Whereas, Electrons are negatively charged particles that orbit the nucleus in shells or energy levels. These shells are arranged at different distances from the nucleus and can hold varying numbers of electrons. 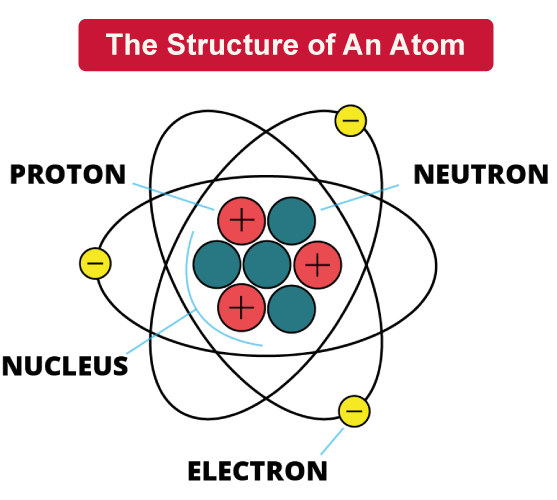
The atomic number and identity of an atom are determined by the number of protons in its nucleus, while the chemical makeup and behavior of an atom are determined by the number of electrons in its shells. Overall, the structure of atoms can be thought of as a tiny solar system, with the nucleus as the sun and electrons as the planets orbiting around it. Atomic NumberThe atomic number is a unique number that represents the number of protons in the nucleus of an atom. It's like a unique ID number that identifies the element. Every element on the periodic table has a unique atomic number, which helps to identify and differentiate it from other elements. For example, hydrogen has an atomic number of 1, which means it has one proton in its nucleus. Carbon on other hand, has an atomic number of 6, indicating it has six protons in its nucleus. Atomic number of an element also determines its chemical properties and how it will react with other elements. Number of neutrons in an atom can vary, but its atomic number always remains same for any given element. In summary, atomic number is a fundamental property of an element that helps us in identifying it and gives it unique characteristics. Isotopes of An ElementIsotopes are atoms of same element that have a different number of neutrons present in their nucleus. This means, isotopes have same number of protons, which determines the element, but different numbers of neutrons, which affects their atomic mass. For example, carbon element has isotopes such as C-12, C-13, and C-14. Six protons and six neutrons make up C-12, six protons and seven neutrons make up C-13, and six protons and eight neutrons make up C-14. Each of these isotopes has a different atomic mass because they have different number of neutrons present in nucleus. Isotopes can be either stable or unstable, and those that are unstable undergo radioactive decay over time. This indicates that as they transform into more stable forms, they emit radiation. While certain isotopes have a very short half-life and degrade extremely quickly, others have a longer half-life and degrade more gradually. Isotopes are used in a wide variety of disciplines. Nuclear medicine imaging in medicine uses isotopes to help identify and treat conditions like cancer. The age of ancient artefacts and fossils can be determined using isotopes in conjunction with carbon dating. Isotopes can be used in geology to date rocks and investigate the Earth's past. Both the development of nuclear weapons and the production of nuclear electricity both use isotopes. Atomic MassAtomic mass, which is typically expressed in atomic mass units (amu) or dalton (Da), refers to the mass of an atom of a chemical element. Atomic mass is calculated by adding mass of protons, electrons and neurons inside atom. One atomic mass unit (amu) or one dalton is equal to one-twelfth (1/12) the mass of a Carbon-12 atom, which is the most prevalent form of the element. In other words, one amu is equal to 1.6605 x 10-27 kilogrammes. Both protons and neutrons have mass roughly equal to one amu. The mass of electrons is negligible, compared to protons and neutrons (0.0005 amu), it can be neglected while determining atomic mass. Hence we can estimate mass of an atom by adding number or protons and neutrons. Take carbon, which has six protons, six neutrons, and six electrons, as an example. Combined masses of carbon's protons, neutrons, and electrons make up the element's atomic mass. As a result, carbon's atomic mass is: (6 protons x 1 amu/proton) + (6 neutrons x 1 amu/neutron) + (6 electrons x 0.0005 amu/electron) = 12.011 amu Hence, atom of C-arbon-12 has atomic mass of about 12 amu. Electronic ConfigurationElectronic configuration is a way of describing how electrons are arranged within an atom or molecule. The negatively charged electrons that round an atom's nucleus are in motion. Each electron in an atom has a certain energy level or orbital assigned to it.The electronic configuration of an atom specifies how many electrons are in each energy level or orbital. Number of electrons in each orbital is often expressed in a shorthand notation. Carbon, which has six electrons, for instance, has the electrical configuration 1s2 2s2 2p2. This means that there are 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and 2 electrons in the 2p orbital. The electronic configuration of an atom determines its chemical properties and reactivity. Atoms with full outer electron shells tend to be stable and unreactive, while those with partially filled outer shells tend to be reactive and form chemical bonds with other atoms. In conclusion, electronic configuration is an important concept in chemistry and physics that helps us understand the behavior of atoms and molecules. Chemical BondingChemical bonding is the process by which atoms combine with each other to form molecules or compounds. It occurs when the outermost electrons, called valence electrons, of two or more atoms interact with each other. When electrons are shared or transferred during these interactions, the atoms are surrounded by a stable configuration of electrons. There are three types of chemical bonds: covalent, ionic, and metallic. In covalent bonding, atoms share electrons to form a molecule. In ionic bonding, one or more electrons are transferred from one atom to another, creating positively and negatively charged ions that are attracted to each other. In metallic bonding, electrons are shared among many atoms, creating a lattice of positive ions surrounded by a "sea" of electrons. 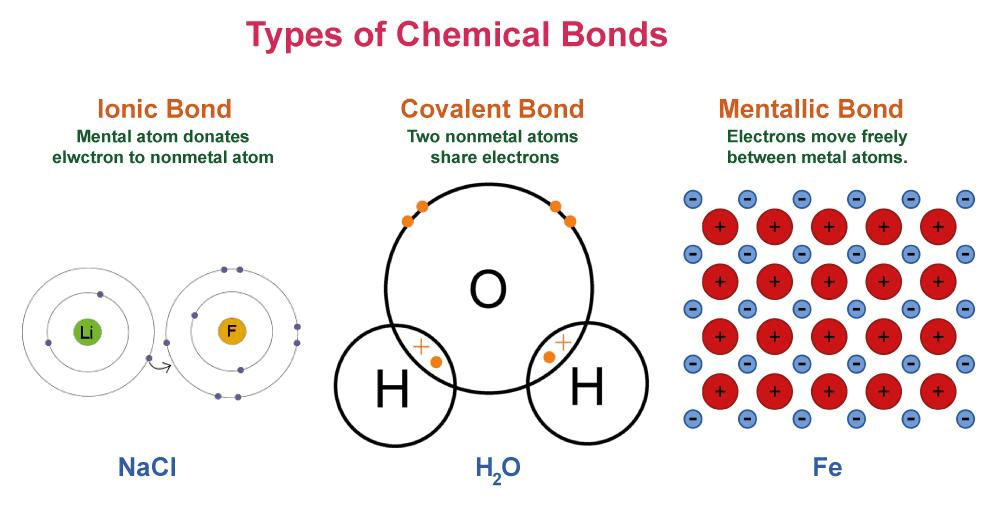
Chemical bonding is essential process in formation of molecules and compounds, and it plays a crucial role in the structure and properties of matter. Subatomic ParticlesThere are numerous other microscopic particles in the cosmos in addition to electrons, protons, and neutrons. Quarks, which make up protons and neutrons, leptons like the electron, bosons, which mediate fundamental forces, mesons, which decay swiftly, baryons formed of three quarks, and antiparticles, which act as the opposite of particles, are a few examples of these particles. Scientists have learned more about the functioning of the universe because of the study of these particles. Applications of Atomic TheoryNumerous real-world applications of atomic physics can be seen in daily life. For instance, it aids in our comprehension of the behavior of various elements and how compounds are created when they mix. It also describes how energy is transmitted between atoms and chemical reactions occur. We have been able to create wonderful technologies that rely on this knowledge of atoms, such as nuclear power plants, X-ray gadgets, and MRI machines, thanks to atomic theory, which explains to us how atoms behave. In conclusion, atomic theory serves as the cornerstone of contemporary science and finds use in a variety of disciplines. ConclusionAtoms are the building blocks of everything in the universe. They determine the chemical and physical properties of all substances. They can combine to form molecules and compounds, and the study of atoms and their behavior is known as chemistry. The structure of atoms resembles a tiny solar system, with the nucleus as the sun and electrons as the planets orbit around it. The study of atoms has led to the development of technologies like nuclear medicine and carbon dating, and understanding their behavior is essential in modern science.
Next TopicBod Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










