Battery DefinitionA battery is an externally connected electrical power source that can be used for electrical power appliances. It is made up of one or more electrochemical cells. The cathode and anode of a battery are represented by their positive and negative terminals, respectively, when it supplies electricity. Electrons will come from the terminal labeled "negative" and travel to the terminal labeled "positive" via an external electric circuit. When a battery is attached to an electrical load outside of it, a redox reaction transforms high-energy reactants into lower-energy goods. The resulting free energy difference is transferred as electrical energy to the load outside the battery. The term "battery," which was once only used to describe a device of several cells, is now also used to describe anything made up of just one cell. 
Alkaline batteries, which are frequently used in flashlights and other portable electronic devices, are an example of primary (also known as "single-use" or "disposable") batteries that are only intended for one use before being discarded due to the irreversible change in electrode materials during discharge. With an applied electric current, secondary (rechargeable) batteries can be repeatedly discharged and recharged; reverse current can restore the electrodes' original chemical makeup. Lead-acid and lithium-ion batteries, which are used in portable devices such as laptops and cell phones, are two examples. Batteries come in a broad range of shapes and sizes, from the tiny cells used to power hearing devices and wristwatches to the enormous banks of batteries the size of rooms that offer backup or power emergencies for telephone lines and computer data centers, which are at the highest end of the scale. As measured by energy per mass, batteries have substantially lower specific energies than typical fuels like gasoline. When it comes to transferring electrical energy into the mechanical effort, electric motors are more efficient than combustion engines in cars, which helps to offset this. What Exactly is a Battery?An electrochemical device that can be charged with an electric current and discharged as needed is known as a battery. A battery can be made up of one or more electrochemical cells. Typically, batteries are constructed of several electrochemical cells coupled to external input and output devices. Small electric devices like remote controls, cell phones, and flashlights frequently use batteries as their power source. The combination of two or more electrochemical cells has always been referred to historically as a battery. However, it's thought that the term "battery" now includes something with only one cell because that's what most people consider a modern battery. The two groups of batteries-primary and secondary-represent a general classification of batteries. Only one charge is permitted for primary batteries. These batteries must be discarded when they are entirely depleted because they are no longer useful. Because of the irreversible nature of the electrochemical reaction that occurs inside primary batteries, this is the most frequent cause for their inability to be recharged. The term "primary batteries" can also apply to use-and-throw batteries, which is significant to note. On the other hand, secondary batteries can be charged and reused for many charging-discharging cycles. The electrochemical reactions that take place inside these batteries are usually reversible. Therefore, secondary batteries are also known as rechargeable batteries. When discharging, the reactants combine to form products, resulting in the flow of electricity. When charging, the flow of electrons into the battery facilitates the reverse reaction, in which the products react to form the reactants. Invention: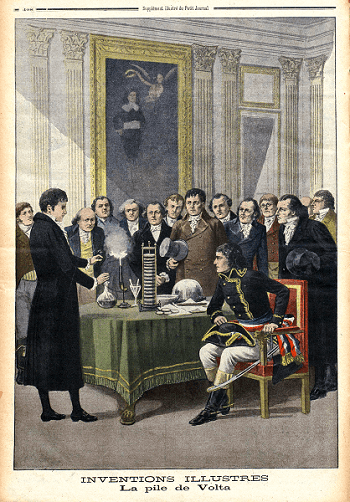
The Baghdad battery, a first-century invention made of a ceramic pot, copper, and iron, was discovered, according to Wilhelm König, head of the Bagdad Museum and Iraq Antiquities Department, in the 1930s. He believed it to be a device for electroplating, but subsequent theories contend that it might have been an electrotherapy device. Using a network of connected Leyden jar capacitors for electrical experiments, Benjamin Franklin coined the word "battery" in 1749. Using the military word for a collection of interconnected weapons, Franklin arranged several of the jars into what he called a "battery." A stronger charge could be stored by increasing the number of holding vessels, and more power would be accessible when discharged. 
Alessandro Volta, an Italian physicist, created and outlined the voltaic pile, the first electrochemical battery, in 1800. This device could generate a constant current for a sizable period and consisted of a stack of copper and zinc plates separated by brine-soaked paper discs. Volta was not aware that chemical processes caused the voltage. He believed that his cells were an endless supply of energy and that the corrosion effects at the electrodes that went along with them were an inconvenience instead of an inevitable result of their operation, as Michael Faraday demonstrated in 1834. Early batteries were excellent for experiments, but in real life, their voltages varied and they couldn't hold a high current for long. The Daniell cell was the first practical electricity generator, developed in 1836 by British scientist John Frederic Daniell. It quickly rose to industry standards and was widely used as a power source for electrical telegraph networks. It comprised a copper pot containing a copper sulfate solution, a zinc electrode, and an unglazed earthenware container containing sulfuric acid. These wet cells employed liquid electrolytes, which might leak and spill if not handled carefully. Many contained their components in glass jars, making them brittle and sometimes deadly. Wet cells were not suited for portable appliances due to these properties. Portable electrical devices became possible at the tail end of the nineteenth century with the development of dry cell batteries, which used paste instead of liquid electrolyte. In the past, wet cells were employed in Hoover tube systems for the "A" battery (to power the filament) and dry cells for the "B" battery (to provide the plate voltage). Future:Annual battery consumption increased by 30% between 2010 and 2018, totaling 180Gwh in 2018. According to conservative projections, the growth rate would be around 25%, with the demand peaking at 2600Gwh in 2030. Cost savings are also anticipated to boost demand even more, reaching up to 3562 GWh. The electrification of transportation and widespread deployment in electricity grids, supported by anthropogenic climate change-driven shifts away from fossil-fuel-combusted energy sources to cleaner, renewable sources and more strict emission regimes, are significant factors contributing to the electric battery industry's high rate of growth. Distributed electric batteries that are connected to smart grids for demand response and used in residential energy storage, such as those found in battery electric vehicles (vehicle-to-grid), are active participants in these systems. The overall usefulness of electric batteries is increased by new reuse techniques, including echelon use of partially used batteries, which also lowers the cost of energy storage and has a smaller impact on pollution and emissions because of the longer lifespan of the batteries. In secondary battery use, car batteries that have lost more than 80% of their capacity, often after 5-8 years, are recycled for backup energy sources or in renewable energy storage systems. Grid-scale energy storage refers to the extensive usage of batteries to collect and store energy generated by the grid or a power station and then discharge that energy afterward to provide electricity and other grid services as required. Smart power supply infrastructures contain significant amounts of grid-scale energy storage, either turnkey or distributed. Chemistry and Principles:Batteries directly transform chemical energy into electrical energy. In many instances, the difference in cohesive or bond energies of the metals, oxides, or molecules participating in the electrochemical process causes electrical energy to be produced. For instance, high-energy metals like Zn and Li can store energy because, unlike transition metals, they are not supported by d-electron bonding. Batteries are made only to allow electrons to flow through the outside portion of the circuit for the energetically advantageous redox reaction to take place. 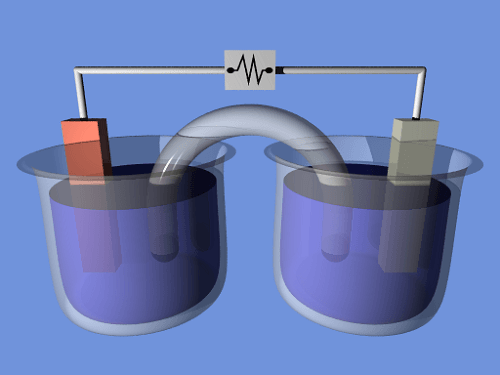
A certain number of voltaic cells make up a battery. Two half-cells comprise each cell, linked together in series by a conductive electrolyte containing metal cations. Electrolytes are present in both cell halves; in one half, anions (negatively charged ions) migrate to the negative electrode, while cations (positively charged ions) migrate to the positive electrode. When metal atoms are oxidized (electrons removed) at the anode, cations are reduced (electrons added) at the cathode. Some cells divide a cell into two halves, each using a different electrolyte. A separator is employed in these cells to keep the electrolytes from mixing, but still, allow ions to go between the two halves and complete the electrical circuit. The electromotive force (emf, measured in volts) of each half-cell is in comparison to a reference value. The difference between the EMFs of the half-cells makes up the cell's net emf. As a result, if the electrodes have emfs, the net emf is defined as the difference between the half-reactions' reduction potentials. The terminal voltage (difference), expressed in volts, is the electrical force applied across a cell's terminals. The open-circuit voltage is the terminal voltage of a cell that is neither charging nor discharging and is equal to the cell's emf. Internal resistance causes the terminal voltage of a cell that is discharging to be smaller than the open-circuit voltage, whereas a cell that is charging has a terminal voltage that is higher than the open-circuit voltage. An ideal cell would maintain a steady terminal voltage until it was completely depleted, at which point it would drop to zero because it had very little internal resistance. The amount of work done by such a cell would be 1.5 joules if it could sustain 1.5 volts and generate a charge of one coulomb. In actual cells, as a cell is discharged, the open-circuit voltage lowers, and the internal resistance rises. The graphs produced by plotting voltage and resistance against time are typically curves; the shape of the curve varies depending on the chemistry and internal layout used. The energy released by the chemical reactions involving the electrolyte and electrodes in a cell determines the voltage between its terminals. The chemistries of alkaline and zinc-carbon batteries are different, but they roughly have the same emf of 1.5 volts; the chemistries of NiCd and NiMH batteries are different, but they roughly have the same emf of 1.2 volts. Lithium cells have emfs of at least 3 volts due to the significant electrochemical potential changes in lithium compound reactions. As long as it contains enough ions to be electrically conductive, almost any liquid or moist material can act as a cell's electrolyte. It is possible to place two electrodes of various metals into a lemon, potato, etc., and generate a small amount of electricity as a novelty or science demonstration. Two coins (such as a nickel and a penny) plus some paper towel dipped in salt water can create a voltaic pile. When numerous of them are stacked in series, they can temporarily replace standard batteries even though a single one produces a very low voltage. Types:Primary and secondary batteriesBatteries are classified into primary and secondary forms: Primary batteries are intended to be used until they run out of power, then thrown away. They cannot be recharged since most of their chemical reactions are irreversible. The battery stops producing electricity and becomes inoperable when the reactant supply runs out. 
The chemical reactions of secondary batteries can be reversed by supplying electric current to the cell, known as recharge. This regenerates them to allow for repeated uses, recharges, and reuses of the initial chemical reactants. Certain primary battery types utilized, such as telegraph circuits, were made functional by switching out the electrodes. Due to the depletion of the active components, electrolyte loss, and internal corrosion, secondary batteries cannot be continuously recharged. Primary batteries, also known as primary cells, can produce electricity after assembly. They are frequently employed in portable equipment with minimal current requirements, are only sometimes utilized, or are located far from a conventional power supply, such as alarm and communication systems, where other forms of electricity are only sporadic and unreliable. Due to the difficulty of reversing chemical processes and the possibility that active ingredients may not return to their original forms, disposable primary cells cannot be reliably recharged. Battery makers do not recommend the attempt to recharge primary cells. Although generally having higher energy densities than rechargeable batteries, disposable batteries do not function well in high-drain applications with loads less than 75 ohms. Alkaline batteries and zinc-carbon batteries are two popular types of disposable batteries. Secondary cells, rechargeable batteries, or simply secondary batteries-are often constructed with active components that must be discharged. They must be charged before use. The chemical reactions that take place during discharge and usage are reversed during (re)charging of rechargeable batteries by the application of electric current. The term "charger" refers to equipment that supplies the proper current. The lead-acid battery, which is a common type of rechargeable battery and is used in a variety of marine and automotive applications, is the most traditional type. This method uses electrolytes in such an open container to ensure the safe release of hydrogen gas created during overcharging, necessitating the maintenance of an upright battery and sufficient ventilation of the surrounding area. It is relatively heavy because of how much electrical energy a lead-acid battery can produce. The lead-acid battery:In 1859, French physicist and inventor Gaston Planté were thought to have developed the lead-acid battery. One of the oldest rechargeable batteries is known to exist in this one. The lead-acid battery is known to have a very high energy-to-volume ratio and a very low energy-to-weight ratio. Yet, the electrochemical cells in this battery are known to have a very high power-to-weight ratio. Their capacity to generate powerful surge currents can be linked to this. These qualities of the lead-acid battery make it particularly desirable for use in motor vehicles and automobiles to supply the high current necessary to start the engine. This trait and the battery's comparatively low price also contribute to its popularity. Lead-acid batteries have several important qualities, including:
The NiCad battery is another name for the nickel-cadmium battery:The nickel-cadmium battery, sometimes known as the "NiCad battery," is a kind of rechargeable battery that uses metallic cadmium and nickel oxide hydroxide as its electrodes. The NiCad battery is well recognized for having a range of discharge rates that depend on the battery's size. A standard AA-sized cell, for instance, has a maximum discharge rate of about 1.8 amperes. A D-size battery, on the other hand, has a discharge rate that can reach 3.5 amps. This is a summary of the NiCad battery's main characteristics:
The lithium-ion battery, or LIB battery, is a type of battery that:The lithium-ion battery, sometimes called a LIB, is a particular kind of secondary battery that can be recharged. It is well known that LIBs are widely used in the aerospace sector and have a wide range of uses in electric vehicle power. There is evidence that lithium ions move from the negative electrode to the positive electrode during the discharging process in batteries (through an electrolyte). It is also known that when charging, these lithium ions travel back. Graphite is typically used as the fuel in the negative electrode of lithium-ion batteries, while an intercalated lithium compound is typically used as the positive electrode. Due to their large energy storage capacity, lack of memory effect (except LFP cells), and low self-discharge, lithium-ion batteries are widely sought after. This is a list of several essential LIB attributes.
Composition:Galvanic cells, electrolytic cells, fuel cells, flow cells, and voltaic heaps are just a few of the various electrochemical cell types developed, each with a different chemical process and architecture. 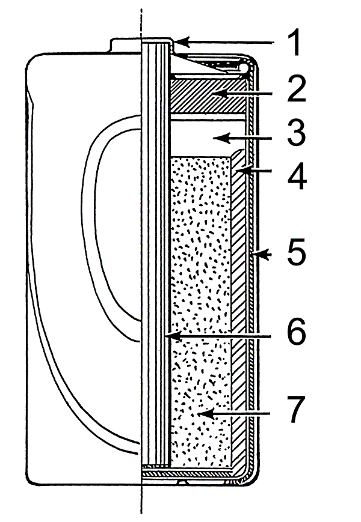
A liquid electrolyte is present in wet-cell batteries. Other names include "flooded cell" and "vented cell" since the interior is completely submerged in liquid. Dry cells were created first, while wet cells were used as an electrochemistry learning tool. They can be constructed using basic laboratory items like beakers to demonstrate how electrochemical cells function. Comprehending corrosion is crucial to use a specific kind of wet cell called a concentration cell. Primary cells (non-rechargeable) or secondary cells may be wet cells (rechargeable). All usable primary batteries, including the Daniell cell, were initially created as open-top glass jar wet cells. The Leclanche cell, Grove cell, Bunsen cell, Chromic acid cell, Clark cell, and Weston cell are further primary wet cells. The first dry cells were modified to use the Leclanche cell chemistry. Wet cells are still used in automobile batteries and also in industries for standby power for switchgear, telephones, or extremely large uninterruptible power supplies, even though gel cells have been used in many places in substitution of wet cells. Typically, lead-acid or nickel-cadmium cells are used in these applications. Molten salt is the electrolyte in molten salt batteries, which can be primary or secondary. They must be well insulated to retain heat since they work under high temperatures. A paste electrolyte, with just the right amount of moisture to permit current passage, is used in dry cells. In contrast to a wet cell, a dry cell may function in any direction without spilling because it has no loose liquid, making it appropriate for portable equipment. In contrast, the original wet cells were often fragile glass jars with lead rods hanging from the open top and required cautious handling to prevent spilling. It took the invention of the gel battery for lead-acid batteries to match the safety and mobility of the dry cell. The zinc-carbon battery, also known as the dry Leclanché cell, is a typical dry cell with the same nominal voltage as an alkaline battery at 1.5 volts (since both use the same zinc-manganese dioxide combination). In a typical dry cell, the zinc anode is often shaped like a cylindrical pot, while the carbon cathode is typically shaped like a center rod. The paste-like ammonium chloride electrolyte is placed adjacent to the zinc anode. The remaining space between the electrolyte and carbon cathode is filled with a second paste made of ammonium chloride and manganese dioxide, the latter of which serves as a depolarizer. Zinc chloride may be used in place of ammonium chloride in some designs. An unassembled reserve battery can be kept for a very long time without being used or providing power (perhaps for years). When a battery is required, it is constructed (by, for example, adding electrolytes), charged, and prepared for use. For instance, the explosion caused by firing a gun may cause a battery for an electronic artillery fuze to be ignited. The battery is turned on and the circuits inside the fuze are powered by the acceleration breaking an electrolyte capsule. Typically, reserve batteries are made for a brief period (seconds or minutes) after extended storage (years). An underwater immersion activates a water-activated battery for use in military or oceanographic equipment. A new type of solid-state battery was created by a team led by John Goodenough, the inventor of the lithium-ion battery. It was announced on February 28, 2017, according to a press release from the University of Texas at Austin. This battery "could lead to safer, faster-charging, longer-lasting rechargeable batteries for handheld mobile devices, electric cars, and stationary energy storage," according to the release. The solid-state battery is also reported to have "three times the energy density," extending its useful life in electric vehicles. Since the method uses less expensive, environmentally beneficial components like sodium that is harvested from seawater, it should also be more environmentally benign. Also, they live substantially longer lives. Sony has created a biological battery that works similarly to how living things make electricity from sugar. Enzymes that break down carbs are used by the battery to produce electricity. As a replacement for lead-acid wet cells, the sealed valve-regulated lead-acid battery (VRLA battery) is well-liked in the automobile sector. A sulfuric acid electrolyte that has been immobilized is used in the VRLA battery to lower the risk of leaking and increase shelf life. VRLA batteries render the electrolyte immobile. There are two varieties:
Wide-sealed "dry cell" varieties of portable rechargeable batteries are also available; these are helpful for laptop and mobile device applications. These cells come in nickel-cadmium (NiCd), nickel-zinc (NiZn), nickel metal hydride (NiMH), and lithium-ion (Li-ion) varieties (in order of decreasing cost and increasing power density). The market for dry cell rechargeables is dominated by Li-ion by a considerable margin. NiCd is still used in power tools, two-way radios, and medical equipment, although NiMH has mostly superseded it due to its higher capacity. Nanoball batteries, which allow for a discharge rate about 100x greater than current batteries, and smart battery packs with state-of-charge monitors and battery protection circuits, which prevent damage on over-discharge, are examples of battery developments from the 2000s. USBCELL, which enables charging an AA battery through a USB connector, is another example of a battery with embedded electronics. Before delivery, secondary cells can be charged due to low self-discharge (LSD). The longest and highest flight driven by solar energy employed lithium-sulfur batteries. Industrial and Consumer Grades:All different kinds of batteries are produced in both consumer and industrial grades. Industrial-grade batteries, which are more expensive, may have chemistries that have better power-to-size ratios, lower self-discharge and, consequently, longer life when not in use, greater leakage resistance, and, for instance, the ability to withstand the high temperature and humidity associated with medical autoclave sterilization. Combination and Administration:The gadget that uses them requires standard-format batteries to be put into the battery holder. When a gadget doesn't use standard-format batteries, they're usually bundled into a special battery pack that may store several cells and has extra features like a battery management system and a battery isolator to ensure the batteries within are charged and drained equally. Sizes:The primary batteries easily accessible to customers range from tiny button cells used in electronic watches to the No. 6 cell used in signal circuits or other long-term applications. Very big batteries can power a submarine, stabilize an electrical system, and aid in balancing peak demands because secondary cells are produced in such massive numbers. In 2017, Tesla constructed the largest battery in the world in South Australia. It has a storage capacity of 129 MWh. A $500 million battery in China's Hebei Province was constructed in 2013 and has a 36 MWh storage capacity. At Fairbanks, Alaska, there was another sizable battery made of Ni-Cd cells. More than a football field, it had a surface area of 2,000 square meters (22,000 square feet) and weighed 1,300 tonnes. Performance, Capacity, and Discharge:The internal chemistry, current drain, and temperature are just a few examples of the many variables that can cause a battery's properties to change throughout a load cycle, a charge cycle, and its lifespan. A battery can't provide as much electricity when it's cold outside. As a result, some car owners add battery warmers, which are little electric heating pads that keep the car battery warm in colder locations. 
The quantity of electric charge that a battery can deliver at its rated voltage is known as its capacity. The cell's capacity increases with the amount of electrode material within it. Although developing the same open-circuit voltage, a small cell has a lower capacity than a larger cell with the same chemistry. Units like the amp-hour (Ah) are used to measure capacity. The current that a new battery can reliably produce for 20 hours at 68 °F (20 °C) while keeping above a predetermined terminal voltage per cell is typically represented as the product of 20 hours multiplied by the capacity of a battery. For instance, a battery with a 100 A/h rating may supply 5 A continuously for 20 hours at room temperature. The amount of the stored charge that a battery can deliver depends on some elements, including the battery's chemistry, the current rate at which the charge is delivered, the necessary terminal voltage, the length of time the battery will be stored, the ambient temperature, and other elements. The capacity decreases with an increasing discharge rate. Due to typically irreversible side reactions that consume charge carriers without creating current, batteries that are kept for an extended time or drained to a tiny percentage of their capacity lose capacity. The term "internal self-discharge" refers to this process. The capacity for successive discharges may also be decreased by different side reactions that can happen when batteries are recharged. When a battery receives enough recharges, it essentially loses all of its capacity and ceases generating electricity. The efficiency of batteries varies due to internal energy losses and restrictions on the pace at which ions may move through the electrolyte. Discharging slowly delivers more of the battery's capacity than quickly discharging does above a minimum threshold. Unless load limits are exceeded, installing batteries with different A-h ratings does not impact the operation of devices rated for a given voltage (although it may change the operation interval). Alkaline batteries see a reduction in total capacity, as do high-drain loads like digital cameras. For instance, a battery rated at 2 A/h for a 10- or 20-hour discharge would not be able to sustain a current of 1 A for the full two hours as indicated by its claimed capacity. The rate at which a battery is being charged or drained is measured using the C-rate. It is calculated by dividing the battery's current through it by the maximum theoretical current draw necessary for the battery to operate at its nominal rated capacity for one hour. It has units h-1. It is uncommon for a battery to operate at its rated capacity in just one hour due to internal resistance loss and chemical reactions within the cells. A battery's capacity is usually discovered at low C-rates, and charging or discharging at higher C-rates shortens the battery's usable life and capacity. Manufacturers frequently publish datasheets featuring graphs of capacity versus C-rate curves. A battery's C-rate rating also shows how much current it can safely supply in a circuit. Rechargeable battery capacity and charge cycle ratings are often based on discharge times of 4 hours (0.25C), 8 hours (0.125C), or greater. Manufacturers may rate certain types for discharge lengths far lower than one hour (1C), such as in a computer uninterruptible power supply, although these types may have short cycle lives. The quickest charging/discharging battery technology as of 2012 was lithium iron phosphate (LiFePO4), which could complete a full discharge in 10 to 20 seconds. Lifespan:There are two definitions of battery life (and its synonym battery lifespan) for rechargeable batteries, but only one for non-chargeable batteries. With rechargeables, it can refer to the number of charge or discharge cycles a battery can withstand before failing to perform properly or the amount of time a gadget can run on a fully charged battery. These two lives are equivalent to non-rechargeables because cells, by definition, only endure for one cycle. (The word "shelf life" refers to how long a battery will maintain its performance between production and use.) All batteries' usable capacity decreases as temperature drops. Although it barely delivers current in the nanoamp range, the Zamboni pile, invented in 1812, has a long service life without refurbishment or recharge. This is in contrast to the vast majority of batteries used today. The Oxford Electric Bell has been running on its original pair of batteries, which are thought to be Zamboni piles, almost constantly since 1840. 
Disposable batteries often lose 8-20% of their initial charge each year when stored at ambient temperature (20-30 °C). This rate, referred to as the "self-discharge" rate, results from "side" chemical reactions inside the cell that don't produce current even when there isn't any load being applied. Although some batteries can be damaged by freezing, side reactions are less common in batteries stored at lower temperatures. A nickel-cadmium (NiCd) battery that has just been charged loses 10% of its charge in the first 24 hours and degrades at a rate of about 10% per month after that. Aging rechargeable batteries, especially nickel-based batteries, self-discharge more quickly than disposable alkaline batteries. Nonetheless, the self-discharge rate of contemporary lithium designs and more current low self-discharge nickel metal hydride (NiMH) batteries have been reduced (but still higher than for primary batteries). Every time the battery is charged and discharged, the active material on the battery plates undergoes a chemical change. Moreover, the active material may be lost due to physical volume changes, reducing the battery's recharging ability. When purchased, the majority of nickel-based batteries are partially depleted; therefore, they need to be charged before use. The discharge rate of more recent NiMH batteries is only 15% per year, and they are ready to use when purchased. Each cycle of charging and discharging results in some degradation. Electrolyte migration away from the electrodes or active material detaching from the electrodes are the two main causes of degradation. High-capacity NiMH batteries (over 2,500 mAh) survive for roughly 500 cycles, while low-capacity NiMH batteries (between 1,700 and 2,000 mAh) can be charged about 1,000 times. NiCd batteries are generally rated for 1,000 cycles before their internal resistance steadily rises over usable levels. Battery lifespan is shortened by rapid charging, which promotes component changes. Overcharging, which can be harmful, is likely if a charger cannot determine when the battery is fully charged. NiCd batteries may experience a capacity reduction known as the "memory effect" if they are used in a specific repeating manner. With a few easy steps, the effect can be avoided. While having a similar chemistry, NiMH cells are less susceptible to the memory effect. Rechargeable automotive lead-acid batteries must withstand the strain from vibration, shock, and various temperatures. Vehicle batteries endure up to six years of regular usage due to these pressures and the sulfation of their lead plates. To increase current, automotive starting batteries (SLI: Starting, Lighting, Ignition) include numerous thin plates. In general, life is longer when the plates are thicker. Before being recharged, they are normally just slightly drained. Lead-acid batteries with "deep cycles," as those found in electric golf carts, have substantially thicker plates. The lead-acid battery's primary benefit is its inexpensive cost, but its primary drawbacks are its large size and weight for a given capacity and voltage. Lead-acid batteries must never be depleted to less than 20% of their capacity because internal resistance might produce heat and harm the battery when recharged. Deep-cycle lead-acid systems usually include a low-charge warning light or a low-charge power cut-off switch to prevent damage that would shorten the battery's lifespan. The battery life can be extended by keeping the batteries in low temperatures, like those in a refrigerator or freezer, which postpones adverse reactions. Alkaline batteries' lifespan can be increased by roughly 5% with such storage, although rechargeable batteries can maintain their charge for much longer, depending on the kind. Batteries must be brought back to room temperature to attain their optimum voltage; discharging an alkaline battery at 250 mA at 0 °C is only 50% as effective as at 20 °C. Manufacturers of alkaline batteries like Duracell do not advise cooling batteries. Hazards:An effort to recharge a primary (non-rechargeable) battery or a short circuit are two common examples of overuse or malfunction that can result in a battery explosion. When a battery is recharged too quickly, pressure can build up inside the battery and eventually cause the case to burst because an explosive gas mixture of hydrogen and oxygen may be formed quicker than it can leave the battery (for example, through a built-in vent). In severe circumstances, battery chemicals could forcefully spray out of the shell and hurt someone. An expert analysis of the issue reveals that this sort of usage "Between the anode and the cathode, lithium ions are transported via liquid electrolytes. A battery cell can short circuit if it is charged too quickly, which can result in explosions and fires". 
Car batteries are most prone to blow up when a short circuit produces extremely high currents. When these batteries are overcharged, hydrogen is produced, which is extremely explosive (because of the electrolysis of the water in the electrolyte). When used normally, the quantity of overcharging is often quite modest and produces minimal hydrogen, which quickly dissipates. On the other hand, while "jump starting" an automobile, the high current can quickly release significant amounts of hydrogen, which can be explosively ignited by a nearby spark, such as when detaching a jumper cable. A battery explosion can result from overcharging, leakage, or irreparable damage, which is the attempt to charge a battery beyond its electrical capacity. The charger or the device where the overcharged battery is later used could potentially sustain damage. As steam accumulates inside the sealed box while burning a battery, an explosion could result. Numerous battery compounds are toxic, corrosive, or both. The chemicals discharged whenever a leakage occurs, whether on purpose or by accident, could be harmful. A zinc "can," for instance, is frequently used in manufacturing disposable batteries both as a reactant and as a container for the other reagents. The chemicals may leak through the remaining cardboard and plastic of the container if this type of battery is overcharged. The equipment that the batteries power may suffer damage or become inoperable due to active chemical leakage. Because of this, several electronic device manufacturers advise removing the batteries from devices that won't be utilized for prolonged periods. As an electrode or electrolytes, several different types of batteries use hazardous substances like lead, mercury, and cadmium. To avoid causing environmental harm, each battery must be disposed of when its life cycle is complete. The electronic trash includes batteries (e-waste). E-waste recycling services recover toxic materials and can be used in new batteries later. An estimated 179,000 tonnes of the almost three billion batteries bought in the US each year end up in landfills. 
If eaten, batteries can be dangerous or even death. Tiny button cells are particularly prone to ingestion by young toddlers. The electrical discharge from the battery during digestion may result in tissue damage; this damage is occasionally severe and might cause death. Disk batteries that have been consumed often only create issues once they get stuck in the digestive system. The esophagus is the most typical location for disc batteries to become trapped, leading to clinical consequences. Batteries that make it through the esophagus are unlikely to land somewhere else. The patient's age and battery size affect how likely a disc battery will become lodged in the esophagus. Batteries smaller than 21-23 mm do not cause issues for older kids. Because the battery's electricity creates sodium hydroxide, liquefaction necrosis could happen (usually at the anode). As soon as six hours after consumption, perforation has happened. Regulation and Legislation:Topics like recycling and safe disposal are covered in laws about electric batteries. The sale of batteries containing mercury was outlawed in the United States in 1996 as a result of the Mercury-Containing and Rechargeable Battery Management Act, which also set standardized requirements for rechargeable battery labeling and required that rechargeable batteries be easily removable. The placement of rechargeable batteries in solid garbage is forbidden in California and New York City. The recycling of rechargeable batteries is conducted by the industry nationally in the United States and Canada, with drop-off locations at neighborhood merchants. In addition to mandating greater battery recycling and encouraging research into better battery recycling techniques, the European Union's Batteries Directive contains similar criteria. Conclusion:Batteries quickly became a necessity for every one of us due to the rapid improvements in technology. All kinds of electronic devices, including phones, laptops, cameras, cars, boats, and medical equipment, are powered by it. Given how reliant we are on these devices, it should be more common than it is to grasp how batteries operate and what they are capable of.
Next TopicBiomedical Waste Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










