What is the Full Form of BCAABCAA: Branched-Chain Amino AcidBCAA stands for Branched-chain amino acid. Branch-chain amino acids (BCAAs) have branching aliphatic side chains (a central carbon atom bound to three or more carbon atoms). The three BCAAs are proteinogenic amino acids: Leucine, isoleucine, and valine. 2-aminoisobutyric acid is one of the non-proteinogenic BCAAs. 
The three proteinogenic BCAAs are one of the nine essential amino acids for humans since they make up three-quarters of the essential amino acids in muscle proteins and 40% of the preformed amino acids required by mammals. Based on the presence of mRNAs that encode enzymes in the metabolic pathway, BCAA synthesis occurs in all parts of plants, specifically within the plastids of the cell pathway. The physiologic and metabolic functions of BCAAs are varied. BCAAs support glucose metabolism, signalling pathways, and protein synthesis and turnover. BCAA oxidation may boost fatty acid oxidation and contribute to obesity. BCAAs play a part in the immune system and brain function physiologically. Immune cells express the dehydrogenase and decarboxylase enzymes, which effectively break down BCAAs, essential for lymphocyte growth, proliferation, and cytotoxic T lymphocyte activity. Finally, BCAAs and aromatic amino acids enter the brain using the same transport protein (Trp, Tyr, and Phe). Once in the brain, BCAAs might contribute to the creation of energy, neurotransmitter synthesis, and protein synthesis. RequirementsThe American Institute of Medicine's Food and Nutrition Board (FNB) established RDAs (recommended daily allowances) in 2002 for essential amino acids. Adults aged 19 and older should consume 42 mg/kg of body weight of Leucine, 19 mg/kg of isoleucine, and 24 mg/kg of valine each day. The equivalent amounts for a 70 kg (154 lb) individual are 2.9, 1.3, and 1.7 g/day. Diets that satisfy or surpass the RDAs for branched-chain amino acids also satisfy or exceed the RDAs for total protein (0.8 g/kg/day; 56 g for a 70 kg individual). ResearchTo treat some cases of hepatic encephalopathy, dietary BCAAs have been employed. Although there is no proof that they improve mortality rates, nutrition, or overall quality of life, they may have the impact of reducing the symptoms of hepatic encephalopathy. Therefore, more research is required. According to certain studies, BCAAs may be connected to the high prevalence of amyotrophic lateral sclerosis (ALS) among professional American football players and Italian soccer players. However, any connection between BCAAs and ALS has not yet been conclusively proven. 
BCAAs generated cell hyper-excitability similar to that typically seen in ALS patients in mice experiments. According to the theorized underlying mechanism, hyperexcitable cells absorb more calcium, which causes cell deaths, especially in neuronal cells with a very low calcium buffering capacity. Even though BCAAs can cause hyperexcitability comparable to that seen in animals with ALS, there is currently no evidence that a diet rich in BCAAs, consumed over an extended period, can cause ALS symptoms. BCAAs may have a role in the etiology of obesity and diabetes because blood levels of these amino acids are higher in obese, insulin-resistant people and mouse and rat models of diet-induced diabetes. BCAA-restricted meals increase glucose tolerance and leanness in mice of normal weight, restore insulin sensitivity and normal body weight in mice with obesity, and increase insulin sensitivity in obese rats. These effects of BCAA restriction in lean and obese mice are mediated by isoleucine and valine rather than leucine restriction. In flies, restricting dietary BCAAs lengthens life, but in mice, restricting dietary BCAAs lengthens male life and reduces frailty but does not lengthen female life. Dietary supplementation with BCAAs alone shortens life span and encourages obesity in mice. Nevertheless, consuming Mice's longevity is increased by an essential amino acid supplement rich in BCAAs. SynthesisThe enzymes threonine dehydrogenase, acetohydroxyacid synthase, ketoacid reductoisomerase, dihydroxy acid dehydrogenase, and aminotransferase all play significant roles in the parallel synthesis routes for isoleucine, valine, and Leucine. Threonine is deaminated and dehydrated to 2-ketobutyrate and ammonia by threonine dehydrogenase. Together, isoleucine and threonine dehydrogenase create a negative feedback loop. The first enzyme in the parallel pathway, acetohydroxyacid synthase, carries out the condensation reaction in both phases, converting pyruvate to acetoacetate in the valine and pyruvate and 2-ketobutyrate to acetohydroxybutyrate in the isoleucine pathway. The acetohydroxy acids from the preceding phase are then reduced by ketoacid reductoisomerase to produce dihydroxy acids in the valine and isoleucine pathways. The dihydroxy acids in dihydroxy acid dehydrogenase are transformed into the next action. Amino transferase completes the last stage of the parallel route, producing the final valine and isoleucine products. Leucine is produced from 2-oxolsovalerate by a set of four more enzymes: isopropyl malate synthase, isopropyl malate isomerase, isopropyl malate dehydrogenase, and aminotransferase. DegradationLeucine, isoleucine, and valine degradation are also shown in the methionine degradation process. 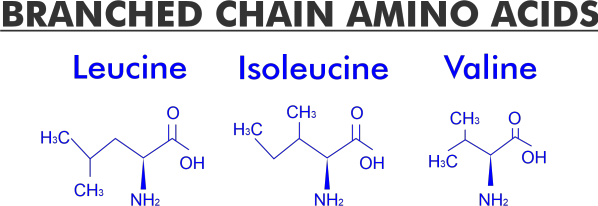
The branched-chain alpha-keto acid dehydrogenases complex breaks branched-chain amino acids (BCKDH). However, the unregulated activity of this complex results in a lack of branched-chain keto acid dehydrogenase kinase. The disorder is known as maple syrup urine disease because a lack of this complex causes an accumulation of the branched-chain amino acids (Leucine, isoleucine, and valine) and their harmful by-products in the blood and urine. The BCKDH complex transforms branch-chain amino acids into acyl-CoA derivatives, which are then transformed into either acetyl-CoA or succinyl-CoA that enter the citric acid cycle. Branched-chain aminotransferase and 3-methyl-2-oxobutanoate dehydrogenase are the involved enzymes. Infection with Maple Syrup UrineIn a model for rats, Acute BCAA administration promotes DNA damage in the brain's hippocampus region in maple syrup urine sickness. The breakdown process for BCAAs is depicted in the Figure below, and the crucial part that insufficient BCKDH plays in maple syrup urine illness is highlighted. 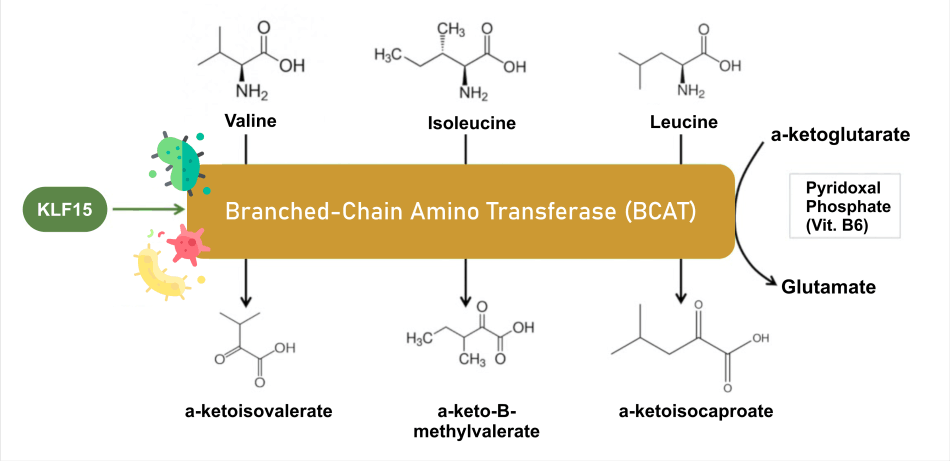
Compared to acute treatment, chronic administration of BCAAs exacerbated DNA damage in the striatum and hippocampus of the brain. Antioxidant therapy stopped DNA deterioration in these brain areas, indicating that BCAAs may damage DNA by causing oxidative stress. Cellular SignallingBCAAs are largely oxidized, whereas most amino acids are oxidized in the liver, skeletal muscle, and other peripheral tissues. The effects of BCAA treatment on rat diaphragm muscle growth were investigated, and it was shown that not only does a BCAA mixture alone have the same effect on growth as a full mixture of amino acids, but a BCAA-free amino acid mixture had no effect at all on the growth of rat diaphragm muscle. Leucine alone seems to be almost as effective as the full mixture of BCAAs, while administration of isoleucine or valine alone had little impact on muscle building. Leucine increases the formation of the eIF4F complex and indirectly activates p70 S6 kinase, both of which are necessary for mRNA binding during translational start. It has been demonstrated that P70 S6 kinase, a component of the mTOR signalling system, enables the adaptive hypertrophy and recovery of rat muscle. Thirty minutes after the initiation of the protein infusion, protein synthesis is stimulated in the at-rest state, and it remains raised for an additional 90 minutes. When Leucine is infused into skeletal muscles at rest, p70 S6 kinase is phosphorylated, leading to a six-hour stimulatory impact and enhanced protein synthesis. Without BCAA delivery, resistance training does not influence mTOR phosphorylation and even decreases Akt phosphorylation after a resistance exercise session. It was shown that p70 S6 kinase was partially phosphorylated. Following a training session, BCAAs were given, and adequate phosphorylation of S6 and p70 S6 kinase revealed activation of the signalling cascade. Role in Type 2 Diabetic MellitusHigh Leucine indirectly involves the mTOR signalling pathway, which is started by high blood glucose levels. The mTOR pathway is involved in cell signalling and beta-cell proliferation, resulting in insulin secretion. mTOR initiates signalling for beta cell proliferation and insulin release in response to the interaction of glucose, Leucine, and other activators. Leucine, in higher amounts, stimulates the mTOR pathway, activating S6 kinase, which then inhibits the insulin receptor substrate through serine phosphorylation. In the cell, the mTOR complex's increased activity eventually prevents beta cells from releasing insulin, and S6 kinase's inhibitory impact produces insulin resistance in the cells, which aids in the progression of type 2 diabetes. The AMP kinase enzyme, which phosphorylates the proteins involved in the mTOR pathway, is activated by metformin as well as causes the mTOR complex to transition from an inactive state to an active state. It has been proposed that metformin functions in the mTOR pathway as a competitive inhibitor of the amino acid leucine. BCAA Supplementation's Effects on ExerciseLactic acid overproduction affects how glucose is metabolized. Glucose levels decrease as a result of BCAA's insulin-like impact on glucose. When BCAAs are consumed before exercise, skeletal muscle can oxidize them and utilize them as energy, minimizing the need for the liver to boost the rate of glycogenolysis. The conversion of pyruvate molecules from glucose metabolism into lactic acid during anaerobic exercise can result in metabolic acidosis with pH levels as low as 6.4. to halt to prevent pH from further lowering. Lactic acid levels in the muscle have been demonstrated to decrease with BCAA supplementation, allowing glucose metabolism to proceed. Reduced glycogenolysis rates in the liver and, thus, decreased plasma glucose levels are the effects of this. However, studies on the long-term effects of BCAAs on blood glucose levels have revealed that regular BCAA intake does not significantly affect blood glucose levels outside of exercise. Additionally, recent research has demonstrated that BCAAs lower blood levels of circulating free fatty acids (FFA). Tryptophan and FFAs fight for binding sites on albumin, and as more free tryptophan is bound by albumin when FFA levels in the blood are reduced, free tryptophan also declines. Exercise raises the amount of free tryptophan that enters the brain, which raises the level of 5-hydroxytryptamine (5-HT, also known as serotonin), which contributes to the feeling of exhaustion. By lowering blood levels of FFAs, BCAAs can lessen the amount of free tryptophan that enters the brain and lessen the feeling of exhaustion brought on by exercise. In rats, a decrease in tryptophan absorption causes a decrease in serotonin synthesis and release.) Low serotonin levels diminish feelings of exhaustion but also cause a lack of attention, poor impulse control, aggressive conduct, and shoddy planning. The decline in serotonin can be as high as 90%. 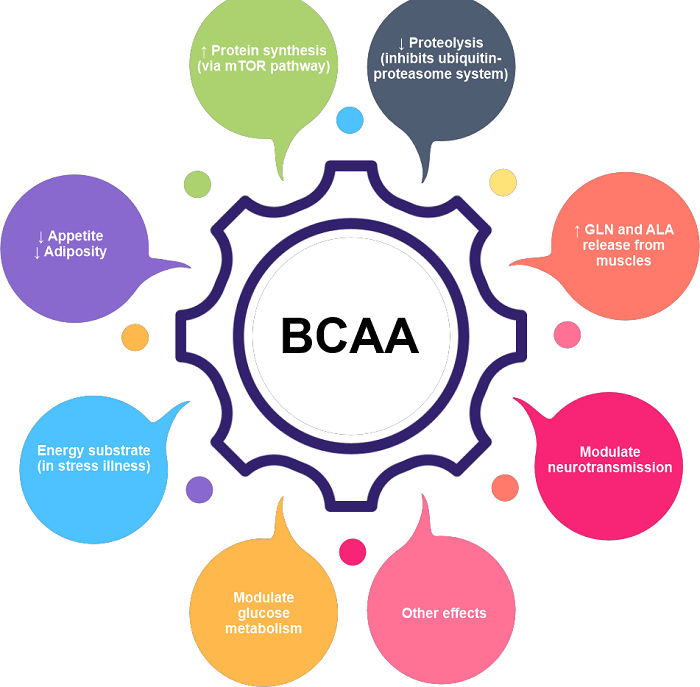
Tyrosine is a different aromatic amino acid from tryptophan, and BCAA prevents it from being absorbed into the brain. Tyrosine's lower uptake inhibits catecholamine synthesis and releases in the brain. Enhanced physical performance is linked to catecholamines. Creatine kinase converts an ATP phosphate group into a phosphocreatine molecule, a marker of muscle injury. BCAA supplementation has been demonstrated to lower creatine kinase levels, which raise intracellular ATP levels and reduce fatigue. BCAAs treat individuals with severe liver illness with movement abnormalities, usually brought on by antipsychotic medicines and compromised brain function. They are frequently utilized to enhance sports performance, avoid tiredness, and minimize. For those with ALS, ingesting BCAAs is not useful. It may worsen lung function and raise the mortality risk for those with this illness. However, more trustworthy data must be used to determine whether utilizing BCAAs for other purposes would be beneficial. Since they immediately promote the metabolic processes necessary to achieve your fitness objectives, BCAAs are a great supplement for active ladies. Although BCAAs can be used by women at any time of the day, before or during workouts is when they are most frequently consumed for two reasons. Branched-chain amino acids are necessary nutrients that assist muscle metabolism and are crucial for constructing protein-rich muscle tissue. Diet and Branched-Chain Amino AcidsThese foods include branched-chain amino acids that you can obtain:
Negative EffectsWhen administered orally: When taken in amounts of 12 grams per day for up to two years, BCAAs are safe. When using BCAAs before or during tasks like driving that call for motor coordination, caution should be exercised. Additionally, BCAAs may result in stomach issues such as bloating, nausea, and diarrhoea. 
Breastfeeding and being pregnant: To determine whether BCAA supplements are safe to use while pregnant or breastfeeding, there isn't enough trustworthy information available. BCAAs are safe for kids when consumed at meal levels. When given to children at higher doses for up to 6 months, they may be safe. Amyotrophic lateral sclerosis (ALS, Lou Gehrig illness): BCAA supplements have been associated with lung failure and greater death rates when used by ALS patients. Until more is known, avoid using BCAA supplements if you have ALS. Branched-chain Ketoaciduria: Consuming BCAAs can cause convulsions and serious delays in a person's mental and physical development. In this case, stay away from BCAAs. Diabetes: Blood sugar levels may be impacted by BCAA supplementation. If you have diabetes and take BCAA supplements, look for low or high blood sugar symptoms and closely check your blood sugar levels. BCAA supplements may influence blood sugar levels, making it difficult to control them before, during, and after surgery. Stop taking BCAA dietary supplements at least two weeks before the operation.
Next TopicFull Form
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










