Carbohydrates DefinitionOne of the three primary ways that our body gets its energy comes from macronutrients called carbohydrates (also called carbs). Since carbon, hydrogen, and oxygen make up their chemical makeup, they are known as carbohydrates. In addition to sugars, fiber, and starches, carbohydrates are also necessary nutrients. In addition to dairy and other dairy products, carbohydrates can be found in cereals, vegetables, fruits, and even milk. These basic food groups are essential and play an important role in a healthy life. During digestion by the digestive system, the food containing carbs is transformed into glucose or blood sugar. Our body's cells, organs, and tissues use this sugar as energy. Our muscles & liver store the excess sugar or energy to be used later. The word "carbohydrate" is derived from the French phrase "hydrate de carbon", which means "hydrate of carbon". This group of organic substances has the general formula Cn(H2O)n and is often termed carbon hydrates. Classification of CarbohydratesCarbohydrates are mainly divided into four major groups, namely monosaccharides, disaccharides, oligosaccharides, and polysaccharides. This classification is made taking into account the degree of polymerization. Let's understand each in detail: MonosaccharidesThe simplest forms of carbohydrates are monosaccharides because they cannot be digested into smaller carbs. The term "mono" illustrates the presence of a single sugar unit. With two or more hydroxyl groups, they are aldehydes or ketones. Unmodified monosaccharides have the typical chemical formula of (C-H2O)n, including "carbon hydrate" in their structure. Monosaccharides are crucial fuel molecules and the foundation of nucleic acids. Dihydroxyacetone and the D- and L-glyceraldehydes are the smallest monosaccharides, with n=3. 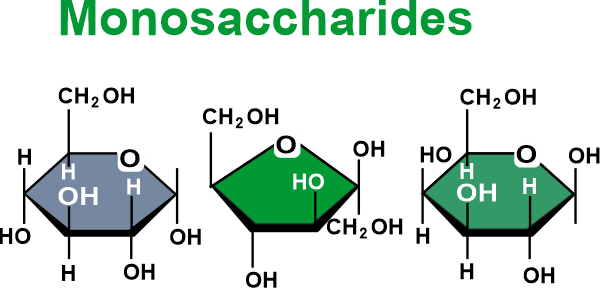
The location of the carbonyl group, the number of carbon atoms present, and the chiral handedness of a monosaccharide are the three features used to categorize them. In the case of an aldehyde carbonyl group, the monosaccharide is an aldose, and in the case of a ketone carbonyl group, it is a ketose. Trioses are monosaccharides that contain three carbon atoms; tetroses are those that contain four; pentoses are those that contain five; hexoses are those that contain six; and so on. This classification system is frequently combined. For instance, the five- and six-carbon aldehydes in ribose and glucose form aldopentoses, and the six-carbon aldehyde in fructose forms a ketohexose (a six-carbon ketone), respectively. Containing the exception of the first and final carbons, all carbon atoms with hydroxyl groups (-OH) are asymmetric, making them stereo centers with two potential configurations apiece (R or S). This asymmetry allows for several isomers for each given monosaccharide formula. Aldohexose D-glucose, for instance, has the formula (CH2O)6, and four of its six carbon atoms are stereogenic, making it one of 24=16 potential stereoisomers according to the Le Bel-van't Hoff rule. There is only one set of potential stereoisomers for the aldotriose glyceraldehydes, which are enantiomers & epimers. The ketose corresponding to aldose glyceraldehydes is 1, 3-dihydroxyacetone, a symmetric molecule without any stereo centers. A typical Fischer projection states that a molecule is a D sugar if the hydroxyl group is located on the right and an L sugar if it is not. This is controlled by the direction of the asymmetric carbon farthest from the carbonyl group. It is important to distinguish the "D-" and "L-" prefixes from "d-" and "l-", which denote the direction in which the sugar rotates planar polarised light. The terms "d-" and "l-" are no longer used in this manner in carbohydrate chemistry. DisaccharidesA disaccharide comprises two linked monosaccharides and is the most basic type of polysaccharide. Suga and lactose are two examples. They are made up of two monosaccharide units joined by a covalent connection called a glycosidic linkage that is produced by a dehydration reaction, which results in the loss of a hydroxyl group from one monosaccharide as well as an atom of hydrogen from the other. Disaccharides have the formula C12H22O11 and are unaltered. Disaccharides come in a wide range of varieties, but only a few stand out. 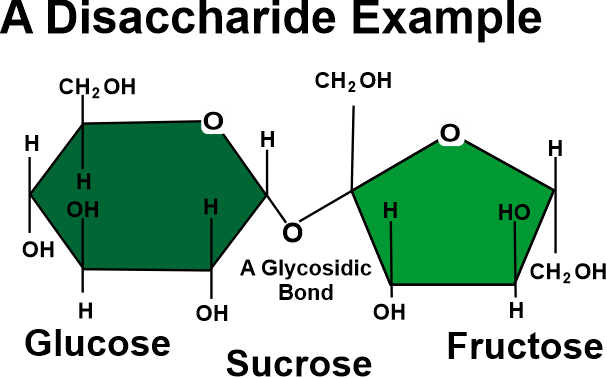
The most prevalent disaccharide and the primary way carbohydrates are carried in plants is sucrose, as shown in the image above. The two molecules that make it up are one D-glucose and one D-fructose. O-?-D-glucopyranosyl-(1?2)-D-fructofuranoside, which is the scientific name for sucrose, denotes four things:
Mammalian milk contains lactose, a disaccharide of one D-galactose and one D-glucose molecule. O-β-D-galactopyranosyl-(1→4)-D-glucopyranose is the legal or systematic term for lactose. Maltose consists of two D-glucose joined by alpha 1,4 glycosidic bonds, and cellobiose, which also consists of two D-glucose linked by a -1,4 bond, are the two prominent disaccharides. Disaccharides can be divided into two categories: reducing & non-reducing disaccharides. OligosaccharidesOligosaccharides are a type of carbohydrate that is created when 2 to 10 monosaccharides come together. Trioses, pentoses, and hexoses are all considered oligosaccharides by this convention. Numerous activities, such as cell adhesion and identification, are possible for oligosaccharides. Oligosaccharide chains are typically found as glycans and are joined to lipids or the side chains of appropriate amino acids in proteins by N- or O-glycosidic connections. The amine nitrogen on the side chain of N-Linked oligosaccharides' pentasaccharides forms a beta connection with the asparagine. Instead, O-linked oligosaccharides are usually attached to the threonine or serine alcohol group of the side chain. 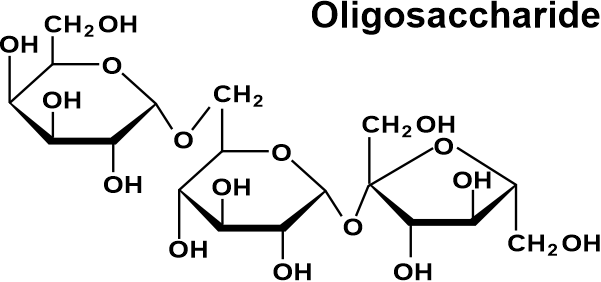
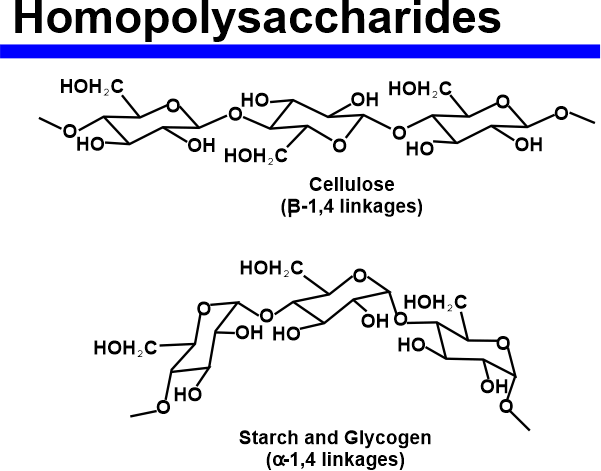
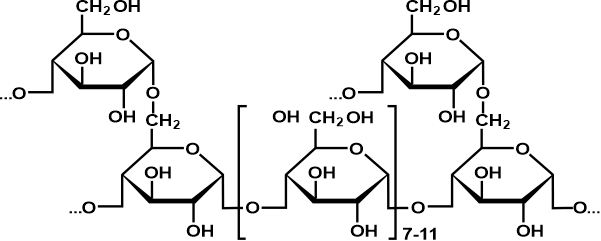
Natural oligosaccharides do not always function as parts of glycoproteins or glycolipids. Some carbohydrates, like the raffinose class, are found in plants as storage or transportation molecules. Others, such as maltodextrins or cellodextrins, are produced by microorganisms that break down bigger polysaccharides like starch or cellulose. PolysaccharidesPolysaccharides typically refer to a chain of over 10 carbohydrates linked together using the glycosidic bond formation. That is the reason they are also known as glycans. Polysaccharides are ubiquitous and primarily take part in the structural or storage functions of organisms. 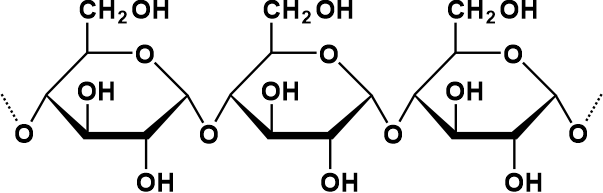
The physical and biological properties of its compounds mainly depend on the constituents involved, as well as their binding or final structure of the reacting molecules and their interactions with the enzymatic machinery. They are often classified based on their functions, the types of monosaccharide units involved, or their origin. Depending on the type of monosaccharides included in the formation of polysaccharide structures, they can be further categorized as homopolysaccharides and heteropolysaccharides. Let us discuss each in brief:
Carbohydrates' PurposesCarbohydrates' primary role in the body and nervous system is to fuel and nourish them. Carbohydrates, which are found in large quantities in grains, fruits, & dairy products, and comprise sugars, starches, and fiber, are one of the basic components of a diet. Starch, simple sugars, complex carbohydrates, and other names are also used for carbs. Carbohydrates also interfere with the metabolism of lipids and stop the body from entering ketosis. Furthermore, they stop proteins from being broken down for energy as the proteins are the body's primary energy source. As a result, an enzyme called amylase helps break down starch into glucose, which ultimately creates energy for metabolism. Various Sources of Carbohydrates
Milk, fruits, & vegetables all typically contain simple sugars enriched with minerals and vitamins. Numerous refined and processed foods, such as white rice, white flour, and sugar, are marketed as "enriched" even though they often lack vital nutrients. Vitamins, carbs, and other organic nutrients can be consumed in their natural forms without causing any harm. Preparation and AnalysisIn general, the polysaccharides, or in which they are found, are broken down with acids to produce monosaccharides. Since it is typically difficult to get sugars in their crystalline form, the crystallization process is typically started by "seeding" a concentrated sugar solution with crystals. The methods used to separate monosaccharides are mostly determined by their chemical and physical properties. Chromatographic techniques are frequently used. Using methods that exploit size, alkaline stability, or a combination of these and other properties of the target molecule, oligosaccharides, and polysaccharides are produced from natural sources. It should be noted that the required molecule often comes in various molecular sizes when an oligosaccharide or polysaccharide is prepared. In contrast to big, intractable molecules like cellulose, the quality of a carbohydrate preparation, typically based on an examination of its composition, is more readily proven for monosaccharides and disaccharides.
Next TopicDemand Definition Economics
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share