Carbon CompoundsCarbon is a highly reactive element. It forms a large number of compounds with various other elements. The word 'carbon' is derived from the Latin word 'carbo' which means charcoal. Carbon is the fourth most abundant element in the entire universe. In our body, it is the second most abundant element after oxygen. In fact, all organic substances in this world contain carbon in one or more forms. This is the reason carbon is the base of the entire organic chemistry. Carbon is a non-metal element, which is located in the group 14 of the periodic table and is represented by the symbol C. Its atomic number is 6, so it has 6 protons, 6 electrons and 6 neutrons. Carbon can easily combine with other carbon atoms in multiple ways and degrees. It is also small in size, so it fits easily in large molecules. Carbon is mostly found in coal deposits. Carbon-containing compounds outnumber the compounds of other elements. The compounds containing carbon are called carbon compounds. The most common compound formed by carbon is methane (CH4). These compounds that contain carbon and hydrogen are called hydrocarbons. The molecular formula of hydrocarbons can be determined by finding the number of hydrogen atoms needed to satisfy the valency of the carbon atom. For example, in one molecule of Ethane, there are two carbon atoms that need 6 hydrogen atoms (3 for each carbon atom) to satisfy the valency of each carbon atom. Therefore, the molecular formula of ethane is C2H6. Properties of Carbon
The above two properties of carbon (catenation and multiple bond formation) enable it to have allotropic forms as described below; What is allotropy?Allotropy is the property of an element that allows it to exist in more than one form and each form is different from each other physically or all forms have different physical properties (structure, shape, colour, etc.,) but have identical chemical properties. The different forms in which the element exists are called allotropes. For example, carbon also exists in different allotropic forms some of them are as follows; i) Crystalline allotropes of carbon: In crystalline form, carbon has diamond, graphite, and fullerene, etc. as its allotropes. They have different physical structures and properties but have the same chemical properties. ii) Micro-crystalline form (amorphous form): In this form, carbon has coal, lampblack and charcoal as its allotropes. Types of carbon compoundsThe bond formed between carbon atoms in the carbon compounds can be single, double or triple. Based on the number of bonds between carbon atoms, they can be classified into two major types that include saturated and unsaturated carbon compounds. i) Saturated Carbon Compounds In these carbon compounds, the carbon atoms are bonded with each other by single bonds. The most common example of saturated carbon compounds are alkanes, e.g., Ethane (C2H6), an alkane, which is a saturated carbon compound as shown in the below image; 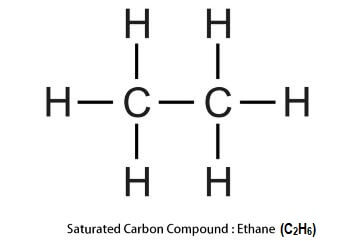
ii) Unsaturated Carbon Compounds In these carbon compounds, the carbon atoms in a carbon compound whether in a chain or ring are joined or bonded together by double or triple bonds. Examples of unsaturated carbon compounds are alkenes and alkynes. In alkenes carbons have a double bond, whereas, in alkynes, they have a triple bond. For example, Ethene (C2H4) belongs to the alkene family so, it has a double bond between carbon atoms. Whereas, Ethyne (C2H2), which belongs to the alkyne family, has a triple bond between carbon atoms. 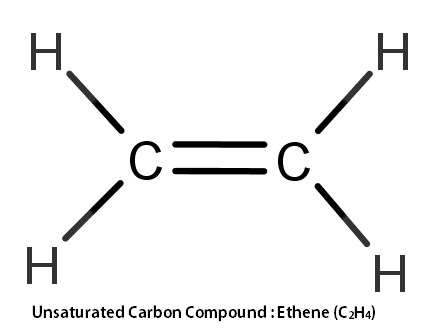
Occurrence of Carbon CompoundsCarbon is one of the most widespread elements. It accounts for around 0.5 percent of the universe mass. Even the solar system is made of a material that is rich in carbon. However, it only makes 0.025 percent of Earth's crust wherein most of it is bound up in rocks and minerals such as chalk and limestone. But, in living organisms, the concentration of carbon is more where it accounts for around one-quarter of atoms in human body tissues. The carbon compounds mainly exist in three different types as described below; i) Straight Chains In this arrangement of carbon compounds, the carbon atoms are bonded to other carbon atoms to form a straight chain without forming any branches. For example, ethane (C2H6) and propane (C3H8). 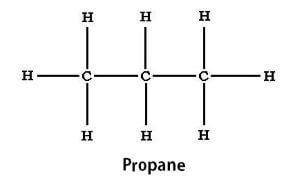
ii) Branches In this arrangement, one of the carbon atoms in the carbon compound is bonded to more than two carbon atoms. The carbon compounds with high molecular weight generally occur in branched form. For example, isopentane (C5H12) as shown below; 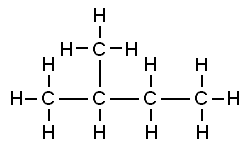
iii) Rings In this arrangement, the carbon atoms are bonded together in such a way that they form closed cycles. So, these carbon compounds are also known as cyclic compounds. For example, cyclohexane (C6H12) as shown below; 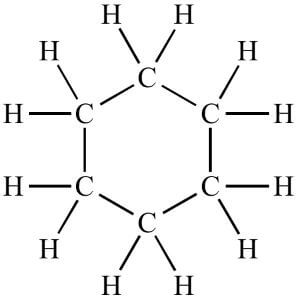
Bonding in Carbon CompoundsThe bonds formed by carbon atoms with other atoms are always covalent bonds in which electrons are shared between atoms participating in the bond formation. Carbon does not form ionic bonds. This is because it has four valence electrons so it is not easy for it to lose all four electrons or to gain four electrons to achieve a stable electronic configuration. So, it forms bonds only with those atoms of elements that are ready to share electrons like carbon. For example, the formation of Methane (CH4): 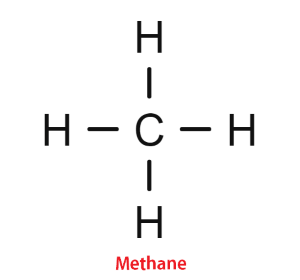
One carbon atom forms bonds with four different hydrogen atoms. Carbon contains 4 valence electrons and Hydrogen has 1 valence electron. So, Carbon needs four hydrogen atoms to share all of its four electrons as its one electron will be shared with one electron of one hydrogen atom only. So, between carbon and hydrogen, there will be one covalent and overall there will be four covalent bonds in one molecule of methane. Physical properties of carbon compounds
Chemical properties of carbon compounds1. Combustion The combustion of carbon compounds or all of its allotropic forms when takes place in air or oxygen produces carbon dioxide, water and heat and light as shown in the below reaction; CH3CH2OH (l) + O2 → CO2 + H2O + heat and light Whereas, when carbon alone burns in air or oxygen, it produces carbon dioxide along with heat and light as shown below; C (s) + O2 → CO2 + heat and light Saturated hydrocarbons when burn in sufficient air or oxygen they produce blue flame (combustion is complete) and produces carbon dioxide, water along with heat and light. Whereas, they burn with sooty flame in a limited supply of air or oxygen due to incomplete combustion as shown below; CH4 + 2O2 →CO2 + 2H2O + heat and light (sooty flame) And, if we talk about the unsaturated hydrocarbon they burn with a yellow smoky flame. 2. Oxidation Carbon and its compounds undergo oxidation or are oxidized in the presence of oxygen. For example, carbon produces carbon monoxide when oxidized as shown below; C (s) + O2 (g) → 2CO (g) Oxidation of carbon compounds like ethanol given ethanoic acid in the presence of oxidizing agents. For example; CH3CH2OH → CH3COOH (Ethanoic acid or Acetic acid) Furthermore, it should be noted that all combustion reactions are oxidation reactions, however, all oxidation reactions are not combustion reactions. 3. Addition reactions These are the reactions that allow carbon to form long chains of atoms. The unsaturated carbon compounds that contain multiple (double or triple) bonds like alkene and alkyne undergo addition reactions to become saturated compounds that contain single bonds. For example, ethene that contains double bonds, when heated in the presence of hydrogen and nickel as a catalyst produces ethane as shown below; CH2=CH2 + H2 + Nickel (catalyst) → CH3-CH3 Similarly, Butyne (unsaturated carbon compound) is converted to Butane as shown below; CH3C?C-CH3 (Butyne) → CH3-CH2-CH2-CH3 (Butane) 4. Substitution reactions Carbon compounds also undergo substitution reactions in which one or more atoms of a compound are replaced by another atom or group of atoms. For example, the functional group of a compound is replaced by a functional group of another compound, as shown in the below reaction; CH3Cl + OH- → CH3OH + Cl- Furthermore, saturated hydrocarbons have low reactivity chemically. So, they are also known as paraffins which means little affinity. However, they tend to undergo substitution reactions under suitable conditions. For example, methane (CH4) when reacts with chlorine in the presence of sunlight the hydrogen atoms of methane are replaced by chlorine atoms as shown below; CH4 + Cl2 → CH3Cl + HCl (methyl chloride + hydrogen chloride) CH3Cl + Cl2 → CH2Cl2 + HCl (methylene chloride + hydrogen chloride) CH2Cl2 + Cl2 → CHCl3 + HCl (chloroform + hydrogen chloride) CHCl3 + Cl2 → CCl4 + HCl (carbon tetrachloride + hydrogen chloride) Uses of CarbonCarbon is widely used in our daily life. Some of its common uses are listed below;
Next TopicTypes of Solids
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










