Carbonic AcidCarbonic acid is an inorganic compound as it lacks a bond between hydrogen and carbon atoms. Its chemical formula is H2CO3. However, it can be written as OC(OH)2 owing to the presence of one carbon-oxygen double bond in its molecule. It is also called acid of air, aerial acid or dihydrogen carbonate. It is a weak acid, which produces carbonate and bicarbonate salts. Further, it is also referred to as a respiratory acid as it is the only acid that is excreted in the gaseous state by the lungs in our body. Carbonic acid is a very common acid owing to the abundance of carbon dioxide and water. It is found only in the form of its salts like carbonates; acid salts like hydrogen carbonates; amines like carbamic acid and as acid chlorides such as carbonyl chloride. Carbonic acid also dissolves limestone to form calcium bicarbonate Ca(HCO3)2. Carbonic Acid Structure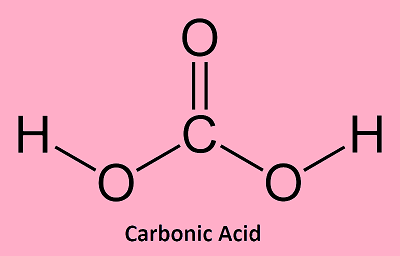
The molecule of carbonic acid (CH2O3) is made of one carboxyl group and two hydroxyl groups. Being a diprotic acid, it can donate two protons. Carbonic Acid OccurrenceIt is found in the blood of humans. Its formation takes place when water gets dissolved with carbon dioxide. The lungs move it out of the body. Besides this, it is also present in coal, meteors, rainwater, calcite, erythrocytes, volcanoes, proteins, plants, and more. Physical Properties of Carbonic Acid
Chemical Properties of Carbonic Acid
Preparation of Carbonic AcidIt is prepared by the dissolution or hydrolysis of CO2 in water. For example, carbon dioxide, when dissolved in water results in the following chemical equilibrium and forms carbonic acid. CO2 + H2O ⇌ H2CO3 However, a small amount of carbonic acid is formed as less carbon dioxide changes into carbonic acid. In industries on a large scale, it is obtained as the by-product of fermentation, fossil fuel burning and other processes. It is also prepared by making strong acid act on calcium carbonate and by allowing the carbon dioxide to pass into the water. The experiment is described below: Reagents required:
Steps:
The chemical reactions that take place in the above method are as follows: i) CaCO3+ HCl → CaCl2+ CO2 ↑+ H2O ii) CO2+ H2O → H2CO3 Uses of Carbonic AcidCarbonic acid offers a wide range of uses. Some of which are as follows:
Importance of Carbonic Acid in BloodThe bicarbonate ion acts as an intermediate for the removal of carbon dioxide from our bodies through the respiratory exchange of gases. The hydration of carbon dioxide is very slow in the absence of a catalyst. But, when carbonic anhydrases, present in the blood are used as catalysts, the reaction rate of hydration increases and thus the conversion of carbon dioxide and water to ions of carbonic acid increases. The bicarbonate anions produced in this reaction get dissolved in the blood plasma. This reaction is reversed in the lungs to form carbon dioxide, which is exhaled out of the body during expiration. Importance of Carbonic Acid in OceansThe excess of carbon dioxide that is released into the atmosphere is absorbed by the oceans. It is believed that due to this absorption the pH of water of oceans has changed by around -0.1. Further, the absorbed carbon dioxide reacts with ocean water to form H2CO3, this process is known as ocean acidification. Health Hazards of Carbonic AcidIt is not toxic for human health as it is naturally found in the human body. However, if someone is exposed to a high concentration of H2CO3 for a longer duration, he or she may experience irritation in the eyes and respiratory tract.
Next TopicTartaric Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










