Carboxylic AcidIt is an organic compound that contains a carboxyl functional group. It is widely found in nature and can also be prepared by humans. Its chemical formula is RCOOH. Carboxylic acid when deprotonates produces anion (R-COO-), which is used in the formation of many salts including sops. Structure of Carboxylic AcidThe general chemical formula is R-COOH wherein R is the part of the molecule to which the carboxyl group is attached. The carbon atom of the carboxyl group is bonded to the oxygen atom by a double bond and bonded to the hydroxyl group by a single bond. 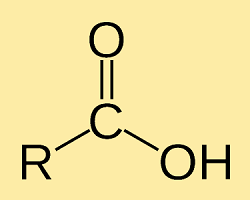
So, it contains a hydroxyl group bonded to a carbonyl carbon. As oxygen is electronegative, the functions group can undergo ionization to release a proton. Some of the carboxylic acids derived from alkanes are methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH) and butanoic acid (C3H7COOH). Physical properties of carboxylic acids
Chemical properties of carboxylic acids
Carboxylic Acid Reactions1. With Metals:When react with metals like K, Na, Mg and Ca, they form salts. The carboxyl group releases a proton and the metal substation occurs during the reaction phase and hydrogen gas is released due to this reaction, which is shown below: 2CH3COOH + 2Na → 2CH3COONa+ + H2 2. With AlkalisThey form salts and water when react with alkalies as shown below: CH3COOH + NaOH → CH3COONa + H2O 3. Reaction with Carbonates and BicarbonatesWhen they react with carbonates and bicarbonates, they form salts, vapor and carbon dioxide gas. This reaction can be used to detect the carboxyl groups. For example, carboxylic acids create effervescence when react with a saturated bicarbonate solution. However, when phenols react with aqueous sodium carbonate they don't produce effervescence. So, this reaction helps identify phenols and carboxylic acids. The reactions take place as follows: 2CH3COOH + Na2CO3 → 2CH3COONa+ + H2O + CO2 2CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 4. Formation of Acid ChloridesCarboxylic acids form their respective acid chlorides when they react with thionyl chloride (SOCl2) and phosphorus pentachloride (PCl5). RCOOH + SOCl2 → RClOCl + SO2 + HCl RCOOH + PCl5 → RCOCl + SO2 + HCl 5. Ester FormationThey form esters with a fruity smell when they are heated with alcohols in the presence of concentrated sulphuric acid or dry hydrochloric acid. For example, the formation of Ethyl acetate (acetate ester) takes place as follows: CH3COOH + C2H5OH →ß CH3COOC2H5 + H2O 6. Amide FormationThey form ammonium salts when they react with ammonia. Further, when ammonium salts are heated they lose water to form amides. The reaction is shown below: CH3COOH + NH3 → CH3COONH4 + heat → CH3CONH2 + H2O 7. Hell-Volhard Zelinsky ReactionIn this reaction, halogenation of a carboxylic acid occurs at the alpha carbon. They form alpha-substituted carboxylic acids whey react with chlorine or bromine molecule. This reaction occurs in the presence of red phosphorus as shown below: CH3COOH → ClCH2COOH → Cl2CHCOOH → Cl3CCOOH 8. Electrophilic Substitution ReactionsAromatic carboxylic acids take part in various electrophilic substitution reactions like nitration, sulphonation and halogenation. It occurs at the meta-position as the carboxyl group is an electron-withdrawing group. The reaction is shown below: C6H5COOH + HNO3 → C6H4 (COOH) NO2 + H2O C6H5COOH + H2SO4 → C6H4 (COOH) SO3H + H2O 9. Friedel Crafts ReactionsAromatic carboxylic acids do not undergo friedel-carfts reactions as the carboxyl group has a tendency to attract electrons. So, due to the deactivation of the benzene ring, it cannot undergo alkylation and acylation. 10. Decarboxylation:Sodium salts of carboxylic acids when used for the distillation of soda-lime results in a decarboxylation reaction to form alkanes. CH3COONa + NaOH → CH4 + Na2CO3 11. Anhydrides FormationTwo carboxylic acid molecules when heated with a dehydrating agent like phosphorus pentoxide lead to the formation of acid anhydrides. 2RCOOH + heat → RCO-O-RCO + H2O Uses of Carboxylic Acids
Next TopicFatty Acids
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










