Catalysts DefinitionCatalysts are chemicals that change the course of a reaction to change the rate at which it occurs. A catalyst is typically employed to accelerate or raise the reaction rate. In essence, catalysts promote molecular interaction and streamline the entire reaction cycle. The following are some of the key characteristics of catalysts:
Catalysts come in three different forms: Solid, Liquid, and Gaseous. Metals or their oxides, such as sulfides and halides, are among the solid catalysts. Semi-metallic substances such as silicon, aluminum, and boron are also employed as catalysts. The same is true for employing pure liquid and gaseous elements as catalysts. These substances are occasionally combined with the appropriate solvents or carriers. A catalytic reaction takes place in their system and uses a catalyst. In other words, a catalyst and a reactant will undergo a chemical reaction to produce catalytic action. As a result, chemical intermediates are created, which can then rapidly react with one another or another reactant to produce a product. However, the catalyst is renewed when the reaction between the chemical intermediates and the reactants occurs or takes place. The reaction modes between the catalysts and the reactants usually vary widely, and in the case of solid catalysts, it is more complex. Reactions can be acid-base reactions, oxidation-reduction reactions, coordination complexes formation, as well as the production of free radicals. For solid catalysts, surface properties, and electronic or crystal structures greatly influence the reaction mechanism. Some solid catalysts, such as Polyfunctional catalysts, can have several reaction modes with the reactants. 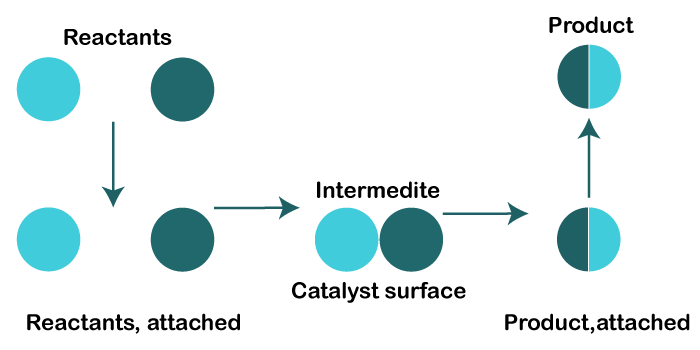
Samples of Different Catalyst TypesDepending on the needs or requirements of the chemical process, many types of catalysts may be used. The list is as follows: 1. Positive catalysts- Positive catalysts are those that speed up a chemical reaction. Decreasing the activation energy barriers speeds up the reaction so that many more reaction molecules are transformed into products, increasing the percentage of product yield. Example of a positive catalyst: Iron oxide works as a positive catalyst in Haber's NH3 preparation process, increasing ammonia production despite decreased nitrogen reactivity. 2. Negative catalysts- Negative catalysts are catalysts that slow down reactions. Raising the activation energy barrier reduces the number of reactant molecules that may be converted into products, the reaction rate is slowed down. An illustration of a negative catalyst is using acetanilide to slow the breakdown of hydrogen peroxide into water and oxygen. Acetanilide functions as a negative catalyst to slow down the rate of hydrogen peroxide breakdown. 3. Accelerators or promoters- Promoter or accelerator is a term used to describe a material that accelerates the activity of the catalyst. For instance, in Haber's process, molybdenum or a combination of potassium and aluminum oxides operate as promoters. 4. Catalyst inhibitors or poisons- Catalyst poisons or inhibitors are substances that lower a catalyst's activity. Example: The catalyst palladium is poisoned with barium sulfate in a quinolone solution to inhibit the hydrogenation of an alkyne to an alkene at the alkene level. Lindler's catalyst is the name given to the catalyst. Units"Katal" is the derived SI unit for describing a catalyst's catalytic activity. Moreover, it is measured in moles per second. If we were to explain a catalyst's productivity, the turnover rate or TON would do. The turnover frequency (TOF), which is TON per time unit, can be used to describe the catalytic activity. Its biochemical equivalent is the enzyme unit. CatalysisCatalysis is the phenomenon that occurs when a catalyst is employed to speed up a chemical reaction. What kinds of Catalysis Exist?There are three different forms of catalysis depending on the substance's composition and physical condition during the chemical reaction;
1. Heterogeneous catalysisThe reacting chemicals in a reaction and the catalyst used in that reaction are not in the same state of matter in this sort of catalysis. Example 1: Making ammonia using Haber's method. A compressor is used to compress pure, dry nitrogen and hydrogen gases in a 1:3 ratio while maintaining a high pressure of 200-230 atmospheres. Iron oxide is employed as a catalyst in this procedure. In a process where the reactants are in a gaseous state, it is a solid oxide. Under the pressure of an iron oxide solid, nitrogen (g) and hydrogen (g) react to generate ammonia (g), making this a heterogeneous catalysis. Example 2: The contact procedure used to manufacture sulfuric acid. Sulfur dioxide oxidation is a crucial phase in this process. Sulfur dioxide is gas, oxygen is a gas, and vanadium pentoxide is a solid catalyst in this oxidation. Reactants and catalysts in this process are in various states of matter. Heterogeneous catalyst mechanism: In addition to adsorption, heterogeneous catalysis also involves the production of intermediate compounds. On the catalyst's surface's activation center, the reactant molecule is adsorbed. They come together to create an intermediate chemical called an activated complex. As this molecule breaks down, goods are produced. When the goods are created, they immediately desorb from the surface. The production of intermediate compounds and product dissociation in heterogeneous catalysis follows initial reactant adsorption on the catalyst surface. Given below illustrates the hydrogenation of ethene into ethane on the nickel surface:
2. Homogeneous catalysisHomogeneous catalysis is the process by which the reactants and the catalyst being used in the reaction are both in the same state of matter. Homogeneous catalysts operate in the same step as the reactants. Typically, substrates and homogeneous catalysts are dissolved in a solvent. One instance of homogeneous catalysis is the impact of H+ on the esterification of carboxylic acids, such as the creation of methyl acetate from acetic acid and methanol. High-volume operations like hydroformylation, hydrosilylation, and hydrocyanation call for a homogenous catalyst. For inorganic chemists, homogeneous catalysis and organometallic catalysts are frequently used interchangeably. Yet, as shown by the employment of cobalt salts to catalyze the conversion of p-xylene to terephthalic acid, many homogeneous catalysts are not organometallic. While small organic molecules without metals may also display catalytic capabilities, as is clear from the absence of transition metals in many enzymes, small organic molecules with metals frequently receive much attention in the study of catalysis. These organocatalysts are competitive with traditional metal-based catalysts and were dubbed a "new breed" in the early 2000s. Compared to transition metal(-ion)-based catalysts, organic catalysts typically require a greater load (catalyst quantity per unit of reactant, expressed in mol % of the material). Still, because these catalysts are typically sold in bulk, costs can be kept down. Example 1: Ethyl acetate hydrolysis in the presence of diluted acid The liquid ethyl acetate has an ester functional group in it. It produces ethyl alcohol and acetic acid when it combines with water in diluted sulfuric acid, a liquid. CH3COOC2H5+H2OCl Reactants and catalysts are in the same state of matter in the process above. It is hence homogenous catalysis. Example 2: The lead chamber process's oxidation of sulfur dioxide. Sulfuric acid is produced via the lead chamber technique. Nitric oxide gas catalyzes this process. Because NO, the catalyst, SO2, and O2 are all gases in the reaction described above, homogeneous catalysis occurs. Homogeneous Catalysis Mechanism: Using the intermediate compound formatter hypothesis, homogeneous catalysis occurs. Let's think about how the lead chamber mechanism converts SO2 into SO3. Nitric oxide gas catalyzes this. This NO interacts with SO2 to produce SO2 and an intermediary molecule called "NO2." First step: Nitrogen dioxide is created when nitric oxide and oxygen interact (NO2). This NO2 interacts with SO2 to produce sulfur trioxide and NO as an intermediate molecule. Intermediate compound: 2NO(g) + O2(g) 2NO2(g) 2SO3(g) + 2NO = 2SO2 + 2NO2 (g) Photocatalysts This phenomenon is known as photocatalysis when a catalyst can absorb light, such as visible light, and be promoted to an excited state. 3. AutocatalysisThere is no special catalyst added to the autocatalytic reaction. Instead, one of the products functions as a catalyst to speed up the production of other compounds. Example 1: Arsenic created in the reactor acts as an "autocatalyst" in the decomposition of assent (AsH3). 2As + 3H2 = 2AsH3 As catalyzes this process. Example 2: Oxidation of oxalic acid by KMnO4. Oxalate ions (or oxalic acid) oxidation occurs when permanganate is added to an acidic solution. The reaction results in the formation of Mn2+ ions, and it auto-catalyzes the reaction. The reaction rate between Potassium permanganate and acidified oxalate solution is initially slow. Mn2+ ions that are formed during the reaction help increase the reaction rate.
Next TopicCentripetal Force Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










