Chromatography DefinitionChromatography is a laboratory method used in chemical analysis to separate a mixture into its constituent parts. The combination passes through a system (a column, a capillary tube, a plate, or a sheet) in which a substance known as the stationary phase is fixed after being dissolved in a fluid solvent (gas or liquid), called the mobile phase. The components of the mixture travel at varying apparent velocities in the mobile fluid, which causes them to separate because they tend to have varied affinities for the stationary phase and are held for various amounts of time depending on their interactions with its surface area. The differential partitioning between the mobile and stationary phases serves as the foundation for the separation. The differential retention impacts the separation on the stationary phase that results from minute variations in a compound's partition coefficient. 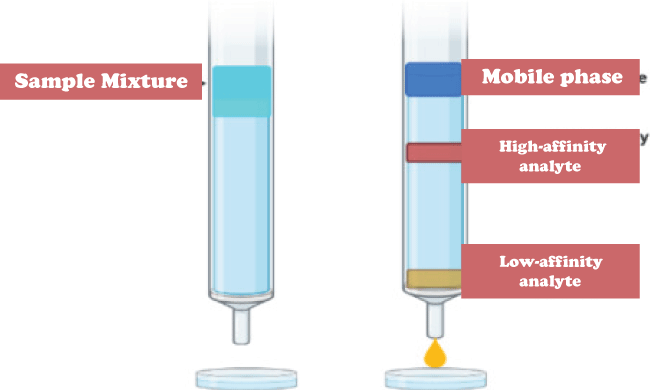
Scientist Mikhail Tsvet, who was born in Italy, invented chromatography for the first time in Russia in 1900. In the first decade of the 20th century, he created the method and coined the word "chromatography," particularly for the purpose of separating plant pigments like chlorophyll, carotenes, and xanthophylls. These components directly influenced the name of the technique since they divide into bands of various colors (green, orange, and yellow, respectively). The approach was beneficial for numerous separation procedures in the 1930s and 1940s because of new forms of chromatography. The work of Archer John Porter Martin and Richard Laurence Millington Synge promoted the quick advancement of various chromatographic techniques, including paper chromatography, gas chromatography, and what would later be known as high-performance liquid chromatography. They pioneered the fundamental concepts and procedures of partition chromatography. Technology has improved significantly since then. The various types of chromatography mentioned below were developed due to researchers discovering that the fundamental ideas behind Tsvet's chromatography could be utilized in numerous ways. Chromatography's technical performance is always being improved, enabling the separation of molecules that are getting closer in similarity.
Different Types Of Chromatography1. Column chromatographyThe stationary bed is contained inside a tube in the separation method known as column chromatography. The mobile phase may have an open, unconstrained passage through the middle of the tube, while the solid stationary phase or support covered with a liquid stationary phase fills the whole interior volume of the tube (packed column) or is focused on or along the interior tube wall (open tubular column). Different retention durations for the sample are determined based on variations in sample movement speeds through the medium. A modified kind of column chromatography known as flash column chromatography was first proposed by W. Clark Still in 1978. (flash). With the exception of driving the solvent through the column with positive pressure, the method is remarkably similar to conventional column chromatography. Better separations than the previous method made it possible to complete the majority of separations in under 20 minutes. Systems can be automated by connecting them to detectors and fraction collectors. Gradient pumps made separations happen more quickly and with less solvent. 
Instead of a solid phase produced by a packed bed, a fluidized bed is used in expanded bed adsorption. This enables the removal of preliminary clearing procedures for culture broths or slurries of broken cells, such as centrifugation and filtering. Numerous DNA-binding proteins have a strong affinity for phosphocellulose, which is used in phosphocellulose chromatography. The amount of salt required to elute a protein depends on how well it interacts with DNA. 2. Paper ChromatographyA little dot or line of the sample solution is applied to a strip of chromatography paper as part of the paper chromatography procedure. A container with a thin layer of solvent and a seal is placed over the paper. Because the cellulose in this paper is polar, less polar chemicals will go further through the combination. 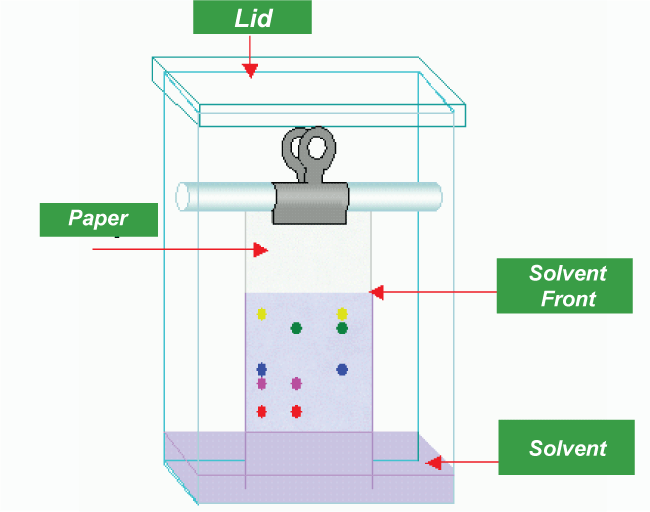
3. Thin-layer Chromatography (TLC)A popular laboratory technique called thin-layer chromatography (TLC) is used to separate various biochemicals based on how attracted they are to the stationary and mobile phases. It resembles paper chromatography in certain ways. Cross-contamination is unlikely because each separation is carried out on a fresh layer. Faster runs, greater separations, better quantitative analysis, and a variety of adsorbents are all advantages over paper. High-performance TLC can be utilized for faster separation with less solvent and even better resolution. 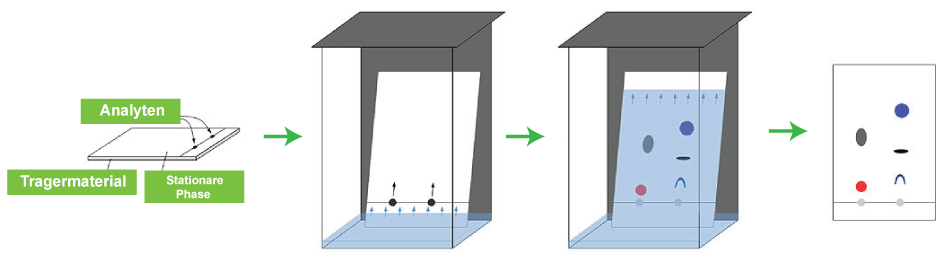
4. Displacement ChromatographyDisplacement chromatography's fundamental tenet is that all molecules with lower binding affinities are displaceable by the displacer, a molecule with a high affinity for the chromatography matrix that efficiently competes for binding sites. The two types of chromatography-displacement and elution-are very different from one another. During elution mode, chemicals frequently leave a column in the form of sharp, Gaussian peaks. A wide separation of peaks, preferably to baseline, is desired for maximal purification. Several variables affect how quickly any component of a mixture descends the column in elution mode. 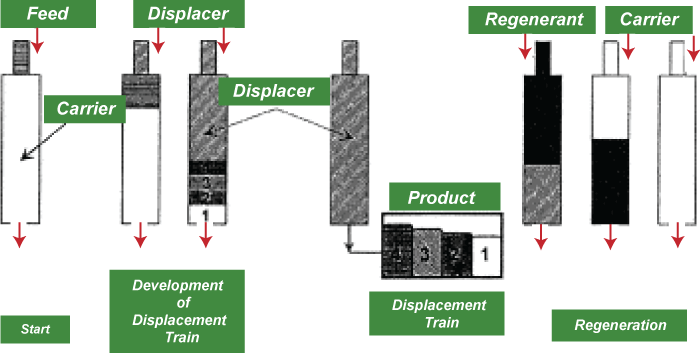
But there must be significant differences in some interaction between the biomolecules and the chromatography matrix for two compounds to move at different rates and so be resolved. To maximize the impact of this variation, operating settings are modified. In many instances, gradient elution and light column loadings are the only ways to separate the peaks' baselines. 5. Gas ChromatographyThe separation method known as gas chromatography (GC), commonly referred to as gas-liquid chromatography (GLC), uses a gas as the mobile phase. Always, a column used for gas chromatographic separation is "packed" or "capillary," depending on the application. Packed columns are the standard workhorses of gas chromatography because they are less expensive, simpler to use, and frequently function well. Although more expensive, capillary columns often provide much better resolution and are increasingly popular, particularly for complicated mixtures. The two types of columns are somewhat combined in SCOT columns, which also feature support particles attached to the column walls but with a liquid phase chemically bound to them. The materials used to construct both varieties of columns are non-adsorbent and chemically inert. Stainless steel and glass are typically utilized for packed columns and for capillary columns, quartz, or fused silica. 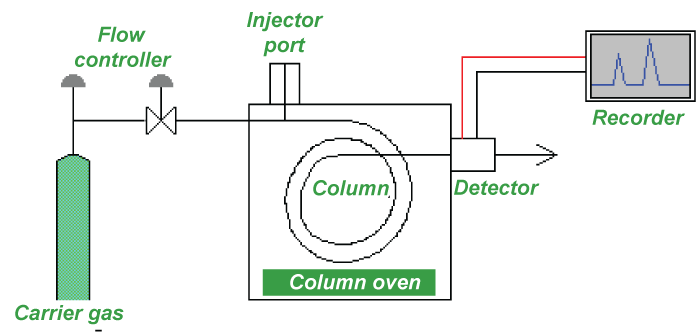
The foundation of gas chromatography is the partition equilibrium of the analyte between a stationary phase that is typically a liquid silicone-based substance and a mobile gas. A capillary column made of glass or fused silica with an interior diameter of 0.53 to 0.18 mm or a solid matrix inside a larger metal tube contains the stationary phase (a packed column). 6. Liquid ChromatographySeparating the molecule where a liquid acts as a mobile phase is called liquid chromatography. It is easily done on either a column or a plane. High-performance liquid chromatography refers to modern liquid chromatography that often uses very fine packing particles and very high pressure (HPLC). 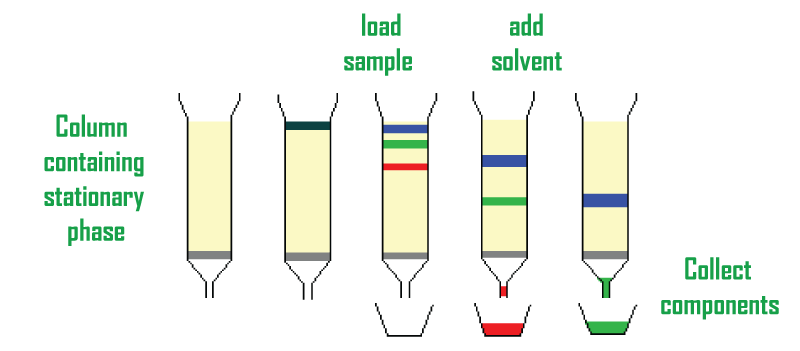
In this type of chromatography, the molecules are passed through a monolith layer which is porous in nature under high pressure. This layer acts as a mobile phase. Monoliths are everlasting blocks of organic or inorganic components that act as "sponge-like chromatographic media.". The polarity of the mobile and stationary phases has historically led to the division of HPLC into two distinct sub-classes. 8. Affinity ChromatographyThe foundation of affinity chromatography is the selective non-covalent interaction of an analyte with particular molecules. Though not extremely sturdy, it is very specific. It is frequently used in biochemistry to purify proteins attached to tags. These tags are typically removed after purification to yield pure protein. Columns are frequently created manually. As a preliminary step, undesirable biomolecules are flushed out using conventional affinity columns. 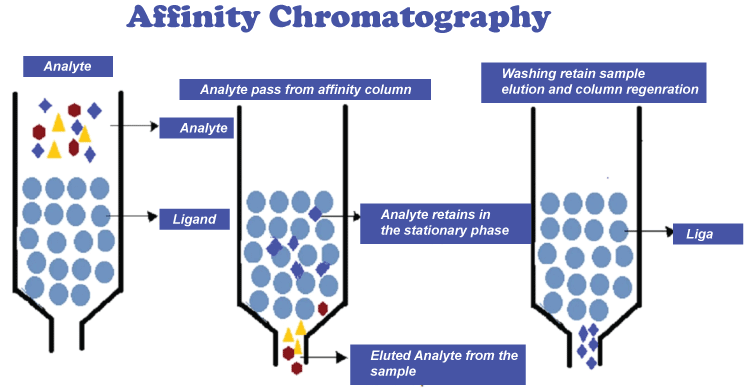
However, there are liquid chromatography methods that do make use of affinity chromatography characteristics. Based on their relative affinities for the metal, the compounds mentioned above can be separated using immobilized metal affinity chromatography (IMAC). These columns can frequently be loaded with various metals to produce a column with a specific affinity. 8. Ion-exchange ChromatographyAn ion exchange mechanism is used in ion exchange chromatography, also known as ion chromatography, to separate analytes according to their relative charges. Although it is typically done in columns, a planar mode can also be effective. The stationary phase in conventional methods is an ion-exchange resin that contains charged functional groups that interact with the compound's oppositely charged groups to retain. Ion exchange chromatography comes in two flavors: anion exchange and cation exchange. While the stationary phase in cation-exchange chromatography has a negative charge and a cation is the exchangeable ion, in anion-exchange chromatography, the stationary phase has a positive charge, and an anion is an exchangeable ion. 9. Size-exclusion ChromatographyIn this type of chromatography, molecules are separated according to their individual size. This technique is also called gel filtration chromatography or gel permeation chromatography. Smaller molecules can fit through the media's pores, and as a result, smaller molecules are caught and removed from the mobile phase's flow. Molecules that are bigger than the packing's average pore size are excluded and essentially don't retain anything; these species are the first to elute. It is typically used as the final "polishing" phase of purification because it is a low-resolution chromatography technique.
Next TopicCommunity Health Nursing Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










