Citric AcidCitric acid is a weak acid, naturally found in citrus fruits. The taste of lemon is actually the taste of citric acid. It is abundantly found in citrus fruits especially in lemons and limes. Though, it is also present in some other fruits and vegetables. Further, it is not only found in citrus fruits but also found in plants and animals in small amounts. A man-made version of citric acid is added to processed foods by manufacturers. Many packaged food items and cosmetics, cleaning products, etc., also contain a manufactured version of citric acid. Citric Acid FormulaThe chemical formula of Citric acid is C6H8O7, which shows it is made of 6 carbon, 8 hydrogen and 7 oxygen atoms. The extended formula of Citric acid is CH2COOH-C(OH)COOH-CH2COOH. 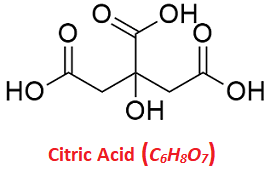
It has a planar structure with three carboxylic acid groups (COOH) and a hydroxyl group (OH). The IUPAC name of citric acid is 2-hydroxypropane-1,2,3-tricarboxylic acid. The other synonyms of citric acid include Citro, and anhydrous citric acid, and more. Further, citric acid aids in generating energy in our bodies and keeps the biosynthesis reactions running in our bodies. It also breaks sugar in glycolysis and provides the energy required in ATP production in the process. Uses of Citric AcidCitric acid has various uses. Its man-made version is widely used in food and drinks, medications, cleaning and personal care products. Some of its main uses are as follows: In Food Industry: It is added to packaged food and drinks. It helps keep packed foods fresh for a longer duration. It can also prevent fresh-cut apples from turning brown. It is also used to give a slightly sour flavor to food items. Alcohol: It can be used to balance the acidity in food or drink. So, sometimes, it is used by winemakers to improve the taste of wines. Medicines: It is also an ingredient in creams as it helps treat skin infections. The other drugs that contain this acid help reduce the acid in the urine and thus helps prevent kidney stones. Citric acid can also be taken for metabolic acidosis, accumulation of acid inside the body. Supplements: It can be taken as calcium citrate supplements, which helps prevent kidney stones. Personal care products: It is used in the formation of 'alpha hydroxy acid' that helps improve skin tone. It is also used in cosmetics like lipsticks, deodorants, lipstick, and hair spray. Household cleaners: Citric acid helps remove hard water buildup, so it is often present in dishwasher detergent. It is also present in other household detergents as it can remove stains and odors. Disinfectants: Citric acid can kill certain bacteria and viruses. So, it is used in insect sprays, hand sanitizers, etc. Environmental cleanup products: These products contain citric acid since it can remove toxins from soil and can even clean nuclear waste. Protective effect in the body: It can reduce the amount of acid in the urine. Emulsifying agent: It acts as an emulsifying agent in ice creams. Furthermore, it is also used for the following purposes:
Citric Acid RisksAlthough the use of citric acid is recognized as safe in food and skin products, according to some experts some more research is needed in this matter. Here are the possible risks associated with the use or intake of citric acid. Skin irritation: The use of citric acid for an extended period of time may cause stinging, swelling or hives. Eye pain: If it enters the eyes, it may cause a burning sensation. In this case, one may flush eyes well with water. Tooth problems: The intake of drinks and food items that contain citric acid can damage the outer layer (enamel) of the teeth. It may lead to sensitive and yellow teeth and cavities. Upset stomach: If taken orally along with medicine, it may cause side effects such as nausea or vomiting. Further, certain drugs when taken with citric acid can cause severe side effects as listed below:
Citric Acid is originally obtained from lemon juice. However, today it is produced from a specific type of mold and used in a variety of applications. Physical Properties of Citric Acid
Chemical Properties of Citric Acid
Preparation of Citric AcidOne can easily buy citric acid from the market as it is a weak acid. You don't need a permit to buy it. Here are the steps to prepare citric acid if you want to prepare it by yourself.
Next TopicHyaluronic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










