Conduction DefinitionIn general, conduction is the process of energy being transferred from one medium particle to another, but in this case, each medium particle maintains its own position. Conduction is primarily understood in Physics or Chemistry to refer to the movement of thermal energy or an electric charge through a substance. Gases, liquids, and solids can all conduct. 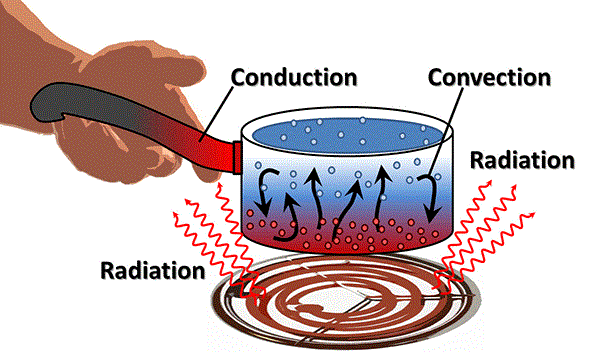
Because the molecules are in direct touch with one another when conduction of heat occurs, the heat energy is often passed from one molecule to the other. However, the molecules' positions remain unchanged. Simply put, they vibrate against one another. Electrically charged particles in the medium move when electricity is being conducted. As a result, ions or electrons are often responsible for carrying and moving the electric current. Types of conduction
A substance with good conduction is referred to as a conductor, whereas a substance with poor conduction is referred to as an insulator. Heat conductionOn an atomic scale, heat conduction can be explained as the physical transmission of heat energy between particles as they come into touch with one another. Despite the fact that the movement of heat within a gas or liquid is more commonly known as convection, this is comparable to how heat is explained by the kinetic theory of gases. The thermal conductivity of a material, which is a measure of how easily heat can be conducted within a substance, determines the rate of heat transfer over time, which is known as the heat current. Less energy is used in the vibrations of the atoms on the cooler side of the bar. The energetic particles interact with nearby iron atoms as they vibrate and transfer some of their energy to those iron atoms. Once the bar is at the same temperature throughout, the hot end starts to lose energy and the cool end to gain energy. This is the thermal equilibrium state. The iron bar is not an isolated system, which is something that the example neglects to mention when thinking about heat transport. In other words, just a portion of the energy from the heated iron atom is transferred to the nearby iron atoms by conduction. The iron bar is also physically in contact with a table, anvil, or another object, as well as with the surrounding air, unless it is suspended by an insulator in a vacuum chamber. The bar will gain energy as air molecules come into contact with it, and they will then transfer that energy away from the bar (though slowly, because the thermal conductivity of unmoving air is very small). Additionally, the bar is so hot that it is glowing, which indicates that part of the heat energy is being released as light. This is just another way the atoms in motion are expending energy. The bar will ultimately cool and achieve thermal equilibrium with the air around it if left unattended. There are two further classes of thermal conduction: 1. Steady-state conduction Assume that the passage of time has no impact on the body's ability to transmit heat. Then, such a definition of heat conduction refers to steady-state conduction. In a steady-state situation, the amount of energy entering and leaving the body is always equal. Generally speaking, the body radiates some of the heat it absorbs. Another component is carried to the next area of the item at the same time. For instance, when one portion of an iron rod is heated, the temperature at several spots along the rod begins to continuously vary. After some time, a state is attained where the temperature is constant throughout the entire object. 2. Non-steady state conduction Transient Conduction is another name for this kind of conduction. The temperature can change at any location inside an item at any given time in this manner of conduction. The fundamental tenet of this is that the body's temperature changes throughout time. This method of conduction typically happens when the body's interior or outside regions experience a change in temperature. When a fresh heat source enters the object, the temperature changes abruptly. Take the starting of a car's engine as an illustration. When the engine is started in this scenario, a fresh heat source is added. However, this conduction phase only lasts for a brief period of time. The steady-state phase first manifests when the engine reaches a certain tolerable temperature. Electrical conductionWhen a substance permits the flow of an electrical current through it, electrical conduction occurs. Whether this is possible relies on the material's physical composition, namely how tightly bound the electrons are, and how readily individual atoms can transfer one or more of their outer electrons to nearby atoms. The electrical resistance of a substance refers to how much it prevents the flow of an electrical current. When chilled to almost absolute zero, some materials lose all electrical resistance and permit electrical current to pass through them without suffering any energy loss. Superconductors are these substances. Sound conductionThe most obvious example of conduction is sound because it is a physical phenomenon produced by vibrations. Atoms in a solid, liquid, or gas vibrate in response to sound, which transmits or conducts the sound through the substance. A sonic insulator is a substance that is perfect for soundproofing since its atoms are difficult to vibrate individually. Examples of conductionIf an ice-cold water glass is left open in the summer, you may have noticed that it slowly warms up. Conduction, of course, is the cause of this. A body and its surroundings transmit heat whenever there is a temperature differential between them. This cycle repeats until the temperature difference between the body and the environment is equal.
Every day in your life, you come across numerous such examples of conduction. Conductors and insulatorsTypically, the handles of culinary utensils are made of materials other than those of the actual utensil. You are better able to avoid getting burned thanks to it. Consequently, these substances are known as weak heat conductors. Insulators are materials that block the passage of heat through them. And conductors are things that allow heat to travel through them. Metals like iron, aluminium, copper, gold, silver, mercury, bronze, steel, etc., as well as non-metals like graphite are a few examples of materials that are effective heat and electrical conductors. Air, oil, pure water, rubber, plastic, diamond, glass, dry paper, and others are examples of insulators. SummaryYou now have a solid understanding of what conduction is and what it means. Conduction is connected to the concepts of heat and temperature in physics and chemistry. The method by which heat or electricity travels through a material is described by the definition of conduction in the dictionary. It might be electrical or thermal. Thermal conduction is the movement of heat, whereas electrical conduction is the movement of electrons. Good conductors are materials that allow heat or electricity to flow through them. Bad conductors, on the other hand, are those who do not let them pass.
Next TopicDefinition of Atomic Number
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










