Difference Between Gas And VapourUnderstanding the key difference between gas and vapour before using either word can help to reduce misunderstandings. The matter may be found in three different states: solid, liquid, and gas. In contrast to liquids, solids have predetermined shapes and volumes (for example Ice). Despite having a fixed capacity, liquids such as water can change form depending on the container. 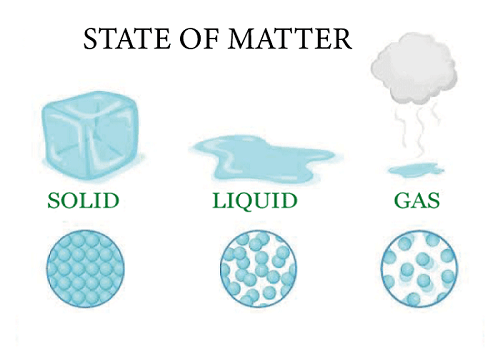
The third and last stage is the gaseous one, which lacks both volume and a defined shape. Examples of gases include oxygen and water vapor. By altering the temperature and pressure, the matter may be changed from one condition to another. The molecules of a material pack in more closely as the temperature are lowered or the pressure is raised. This process enables phase transitions from liquid to solid and from gas to liquid. Phase transitions from solids to liquids as well as from liquids to gases are possible as a result of the molecules spreading apart as pressure and temperature are increased and decreased. Materials have the ability to skip phases and go right on to the next when the regular conditions change. Sublimation is the method of converting a solid straight into a gas without transiting through an intermediary liquid stage. These include things like iodine, camphor, dry ice, and ammonium chloride. GasIn the natural world, gas can be created from a single atom or from a mixture of atoms. It is, nevertheless, a very tiny molecule. For instance, when the halogen group of the periodic table is taken into account, fluorine and chlorine are gases, but bromine is a liquid and iodine is a solid. This is because when an atom gets bigger as it moves down the halogen family tree, huge molecules cannot achieve a state of free mobility because of intermolecular interactions. 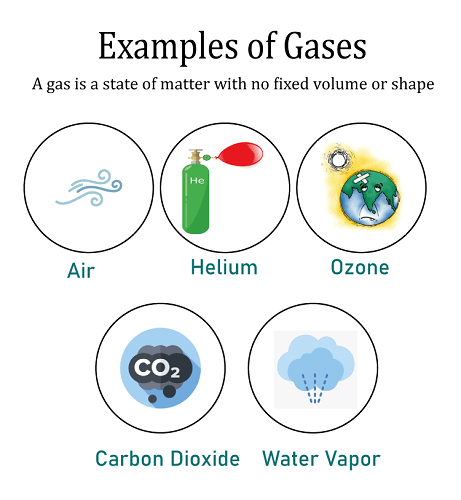
Gas is a material that only exists in one state, the gaseous phase. The thermodynamic state describes this situation. A system's condition may be described in terms of its thermodynamic state using variables like temperature, pressure, and others. Since gas has not undergone a phase shift, it only exists in that state and won't change until particular circumstances are met. Consequently, it is referred to as a monophasic substance. Properties of GasesThey are handy because they can be readily compressed. Gas molecules remain separated in a steady state due to intermolecular attraction. As a result, gases may be compressed easily, and this feature is used to compress gases in research, medicine, and engineering. Compression uses a combination of lowered temperature and raised pressure. The use of air compression is well-illustrated by spray bottles, that compress the gases inside it to make it simpler to spray perfumes when needed. They might all fit inside the container, regardless of how large or little they are. When air is blown into a balloon, the molecules are uniformly dispersed throughout the vessel. VapourA material in the gaseous form that is capable of coexisting with a liquid state is referred to as a vapor. Although this definition can seem a little perplexing, what actually occurs is that vapor and liquid are in balance. The molecules in this liquid are identical to those in the vapor. Vapor is created via a phase transition, and it can change phases again. As a result, it is referred to as a multiphasic substance. 
Unlike gas, the vapor is not a state of matter. Condensation, the production of a liquid drop, and its development are the steps in the conversion of a gas into a liquid. Due to the vapor's mean temperature being below the critical threshold, it is feasible for the vapor and its liquid phase to coexist. The pressure and temperature at which it is impossible to tell if is it a gas or a liquid is known as the critical point. Above the critical point, only gases are possible; a gas and a liquid cannot coexist. For instance, water is a liquid when it is at ambient temperature and a vapor when it is heated to a high temperature. Calculating the quantity of vapour present is done by using partial pressure of gases because it exists in the gaseous form. The Barometer Formula is applied to vapours inside a gravitational field much like it is to regular atmospheric gases. Properties of VapourWhen a material is in a gas phase below its critical temperature, it is said to be in a vapor at that temperature. At that temperature, the same chemical can also exist in a liquid or solid state. A condition of equilibrium will exist between the two phases, if the vapor comes into contact with a solid or liquid phase. A phase of a compressible fluid is referred to as a gas. Fixed gases are those that cannot condense into a liquid or solid at their temperature, such as air at standard ambient levels. Vapor may be produced by a solid or liquid without it having to boil. 
Condensation and cloud formation are well-known effects of vapor. In order to prepare a liquid sample for gas chromatography, it is frequently used to perform the physical operations of distillation as well as headspace extraction. A vapor's component molecules are capable of vibration, rotation, and translational motion. The gas kinetic theory takes these movements into account. Difference Between Gas and VapourOne of the four basic states of matter, along with solid, liquid, and plasma, is the gaseous phase. Because the atoms in gases are in motion and dispersed throughout a container, they can be easily separated from the solid and liquid phases. Despite of their transparency, gas, and vapor are two different phases in which matter can exist. The main distinction between gas & vapor is that although gas can only exist in one physical state, vapor may coexist with various physical states. 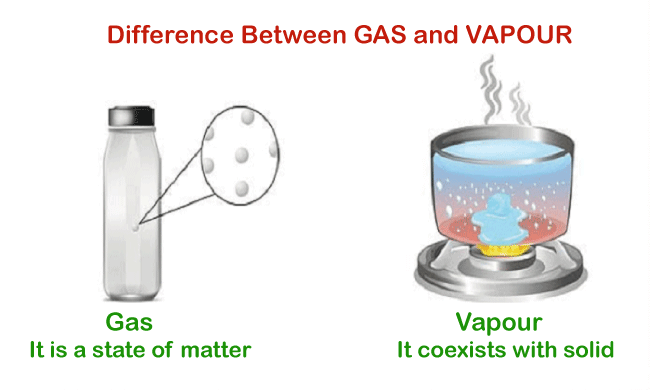
When vapour is studied under a microscope, it exposes a cluster of particles with no recognizable form. Gas does not have a definite form when observed under a microscope. In a vapour, the molecules and atoms are a haphazard collection of particles traveling across space. Unorganized chaos also makes up the atoms and molecules that comprise gas. Gases are a condition of substance, but vapour is not. Gaseous is the condition of substance. Even at temperatures below the boiling point, water vapor is always present around us. SummaryIn contrast to vapor, which is found below the critical point, gas is found above it. Beyond the critical point, no liquid phase is possible. Below the critical point, vapor is present as well. Since vapor may coexist with multiple physical states, it differs from gas in that gas can only exist in one physical condition.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










