Electron Affinity DefinitionWhen an electron joins a neutral atom or molecule in the vapor state to create an anion, energy is released. This energy is referred to as the electron affinity (EEA) of the atom or molecule. X(g) + e? → X?(g) + energy Energy is released upon electron capture, which indicates a positive electron affinity. The definition of an electron's affinities for a surface varies slightly depending on the physical state. 
Electron Affinity Measurement and ApplicationIn a liquid or solid state, interaction with other molecules or atoms would cause the energy levels of the atoms and molecules to change, hence this attribute is only utilized to measure molecules and atoms in the gaseous state. In order to create an electrostatic potential scale for atoms that is equivalent to the mean of the electrons' affinities and ionization potentials, Robert S. Mulliken used a listing of the electron affinities. Theoretical notions like molecular electrostatic potential and molecular hardness also make use of electron affinity. Another illustration is that an electron acceptor is a molecule or an atom that has a higher positive value of electronegativity than another, and an electron donor is one that has a lower positive value. Charge-transfer reactions could occur between them all. Conventional SignsIt is crucial to remember sign in order to apply electron affinities correctly. Any process that releases energy has a change in total energy, E, that is negative and is referred to be an exothermic process. Nearly all non-noble gaseous atoms release energy during electron capture, making it exothermic. The positive values that are listed in tables of EEA are amounts or magnitudes. It is the word "released" within the definition "energy released" that supplies the negative sign to ΔE. Confusion arises in mistaking EEA for a change in energy, ΔE, in which case the positive values listed in tables would be for an endo- not exo-thermic process. The relation between the two is EEA = ?ΔE(attach). If EEA is given a negative value, on the other hand, an electron must expend energy to connect because the negative sign denotes a change in direction. As the electron capture in this instance is an endothermic event, the equation EEA= E(attach) is still true. Negative values often result not only from the nitrogen atom's absorption of a second electron. When one electron is connected, the standard equation for calculating Eea is EEA= (Einitial ? Efinal)attach = ?ΔE(attach) This expression does follow the convention ΔX = X(final) ? X(initial) since ?ΔE = ?(E(final) ? E(initial)) = E(initial) ? E(final). Equivalently, the energy needed to remove an electron from an atom when it is holding a single extra electron, converting the atom into a negative ion, can also be used to describe electron affinity. X? → X + e? Care must be made to apply the right definition to the associated direction, attachment (release), or detachment if the same table is used for the forward and reverse without switching signs (require). These detachment processes are endothermic, or E(detach) > 0, because practically all detachments (required +) some quantity of energy specified on the table. EEA= (Efinal ? Einitial)detach = ΔE(detach) = ?ΔE(attach). The Affiliations Of The Elements To Electrons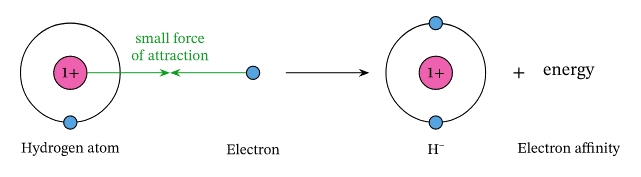
However, though EEA varies considerably among the elements, several trends can be seen. Nonmetals often have a higher positive EEA than metals. The EEA is higher for atoms with anions that are much more stable than neutral atoms. Extra electrons are most strongly attracted to chlorine, while they are least strongly attracted to neon. The noble gas electron affinities could have been positive or slightly negative values since they have not been definitively measured. Before reaching group 18, EEA often rises over a period (row) in the periodic table. This is brought on by the atom's valence shell filling; a group 17 atom gains more energy from an electron gain than a group 1 atom does since the latter has an unfilled valence shell and is less stable. Because group 18 has a full valence shell, any additional electrons are unstable and more likely to be ejected suddenly. Electron Affinities in MoleculesThe intricate relationship between a molecule's electrical structure and its electron affinity. For instance, benzene and naphthalene have negative electron affinities, whereas anthracene, phenanthrene, and pyrene have positive ones. Hexacyanobenzene has a higher electron affinity than fullerene, according to in silico tests. 
In the field of solid-state physics, electron affinity is defined differently than in chemistry and atomic physics. For a semiconductor-vacuum interface (that is, the surface of a semiconductor), electron affinity, typically denoted by EEA or ?, is defined as the energy obtained by moving an electron from the vacuum just outside the semiconductor to the bottom of the conduction band just inside the semiconductor. As an additional electron will naturally move to the bottom of the conduction band in an n-type of semiconductor around absolute zero, this idea is functionally comparable to the definition of electron affinity in chemistry. The analogy is invalid at non-zero temperatures and for other substances (metals, semimetals, and severely doped semiconductors), as an extra electron will typically move to the Fermi level. In any event, the electron affinity number for a solid substance differs significantly from the electron affinity value determined by atomic physics and chemistry for an atom belonging to that substance in the gas phase. As an illustration, the electron affinity of a silicon crystal surface is 4.05 eV, but that of an isolated silicon atom is 1.39 eV. Although they are not the same, a surface's work function and electron affinity are closely related. The work function is the amount of thermodynamic energy that may be produced by isothermally and reversibly withdrawing one electron from a material into a vacuum; this thermodynamic electron, on average, goes to the Fermi level rather than the conduction band edge. While doping can alter a semiconductor's work function, the electron affinity is more closely related to a material constant because it is ideally unaffected by doping. The electron affinity, like work function, is solely a surface property and is dependent on the surface terminating (crystal faces, surface chemistry, etc.). The main application of the electron affinity in semiconductor physics isn't certain in the analysis of semiconductor-vacuum surfaces, but rather in the development of heuristic particle affinity rules for calculating the band bending that takes place at the junction between two materials, particularly at semiconductor heterojunctions and metal-semiconductor junctions. The electron affinity can turn negative under certain conditions. Negative electron affinities are frequently sought to produce effective cathodes that can provide electrons to the vacuum with low energy loss. These structures can be described using band diagrams, where the electron affinity is one of the parameters, using the measured electron yield as a function of different parameters like bias voltage or lighting conditions. Electron Affinity PatternsBecause more electrons are added to energy levels, the nucleus and its electrons are drawn closer together, increasing the electron affinity upward for the groupings and from left to right through periods on a periodic table. Keep in mind that there is less attraction at a distance, therefore adding an electron to the outer orbital releases less energy. Moreover, an element has a higher chance of gaining electrons to form a stable octet the more valence electron it has. The less likely an atom is to gain electrons, the less electron density it contains. Due to the electrons' placement in a higher energy level distance from the nucleus, which reduces their influence on it, electron affinities fall down the groupings and from right to left across the eras on the periodic table. The element should, however, be more stable and have a greater affinity for electrons as the amount of valence electrons increases as one moves down the group, it may be thought. The impact of shielding is not taken into consideration. The shielding effect grows as one decreases the period, which causes the electrons to repel one another. Because of this, as one moves from group to group in the periodic table, the nucleus and electron are less attracted to one another. Initial electron affinities decline as you move down in the group (in the sense that less energy is evolved when the negative ions are formed). It will be necessary to account for fluorine individually because it deviates from this pattern. The amount of energy released depends on how strongly the incoming electron is attracted to the nucleus, which is measured by electron affinity. Nuclear charge, distance, and screening are the same parameters that have an impact on this attraction as they do with respect to ionization energies. More screening electrons are present, which balances out the higher nuclear charge as you move down the group. Regardless of the element being discussed, each electron configuration in effect experiences a pull from the atom's core of 7+. ConclusionLinus Carl Pauling's finding of electron negativity led to the concept of electron affinity, which was found in 1901. The amount of energy shift that occurs when an electron in a gaseous form is introduced to a neutral atom is known as electron affinity. Due to an increase in effective nuclear charge and a decrease in atom size, the electron affinity increases during a period from left to right. Because atoms are getting larger, electron affinity falls within the group. A neutral atom gains energy when an electron is added, and this is why electron affinity is typically exothermic. The secondary electronegativity can be positive because of electron-repelling forces.
Next TopicElectrophoresis Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










