Electronegativity DefinitionElectronegativity refers to the trend of an atom needed in a molecule to attract the pair of electrons it shares with itself, or it is the chemical property that describes the hold of power by an atom in a molecule to attract an already shared pair of electrons towards itself. It does not have any dimensions. It is essential for determining the nature and strength of the bonds between elements. There are several scales to measure Electronegativity, but the most used scale was designed by Linus Pauling. This scale states fluorine as the most electronegative element with a value of 4 and Cesium as the least electronegative element with a value of 0.7. To calculate the polarity of the bonds, subtract the smaller electronegative value from the larger one. Mr. Pauling proposed his concept of electronegativity in the year 1932 in order to explain the covalent bond shared by two distinct atoms. 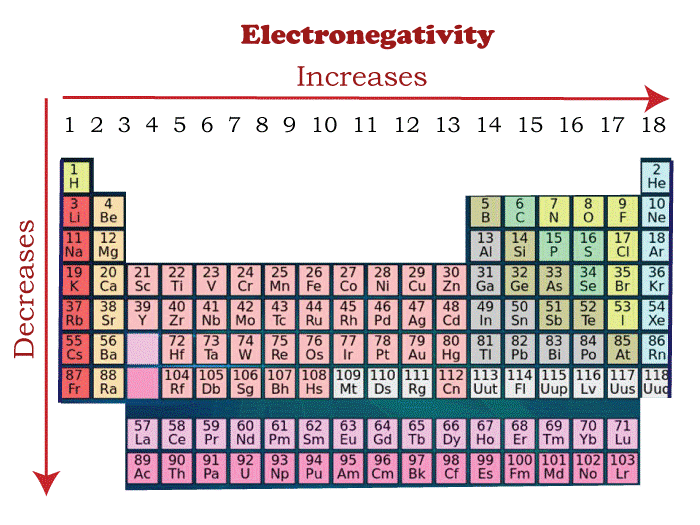
Periodic trends in the electronegativities of the elementAs we move from left to right, nuclear charge increases, and atomic size decreases. Hence, In the modern periodic table, Electronegativity increases across a period. As we move down in a group in the modern periodic table, atomic number and nuclear charge increase. However, the effect of nuclear charge overcomes as new shells add, which drops the electronegativity value. Generally, it is observed that metals exhibit a lower electronegativity value as compared to non-metals. That is why they are called electropositive and non-metals electronegative in their behavior. Electropositive is the exact opposite of electronegative. Therefore, cesium is the most electropositive element. Factors that affect Electronegativity
BondsElectrons in chemical bonds that are attracted to an atom more than the other result in the formation of a polar covalent bond. When the value of electronegativities differs, it indicates that the electrons weren't shared. Therefore, one atom takes the bond electrons from the other and forms the ionic bond. The more difference in the atom's electronegativity values, the more polar the chemical bond created among them will be. ElectropositivityElectropositivity is the element's potential to give or donate electrons and hence become positive ions. It is thus referred to as the antipode of electronegativity. Electropositivity is usually one of the characteristics of metals. The more the metallic character, the more electropositive the element is, which is the rationale behind the electropositivity of alkali metals. Since these metals have only a single ion in their outer shell, which is easy to lose; therefore they have low ionization energies. Electropositivity increases when moving down groups and decreases along periods. Therefore, elements in the upper right of the periodic table are largely electronegative, and elements in the lower left are largely electropositive and least electronegative. Terms related to electronegativity
Next TopicFermentation Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










