Electronic ConfigurationIt refers to the arrangement or layout of electrons in the shells or orbits around the nucleus. The outermost shell or orbit around the nucleus is called valence shell and the electrons present in it are called valence electrons. Rules for electronic configuration or distribution of electronsThere are four energy shells or orbits around the nucleus that are named K, L, M, and N or 1, 2, 3, and 4 starting from the innermost orbit to the outermost orbit. There are three rules for the distribution of electrons in the shells around the nucleus. First rule: The maximum number of electrons in an orbit or energy shell is equal to 2n2 where n = number of shell. For example, for the first orbit (K), n = 1. So, the first shell (energy level or orbit) which is indicated by 'K' can have a maximum of 2 electrons as 2n2 = 2, where n =1. Similarly, the second shell which is indicated by 'L' can have a maximum of 8 electrons (2n2 = 8), where n =2. Similarly, the third shell which is indicated by 'M' can have a maximum of 18 electrons (2n2 = 18), where n =3. Now, the fourth shell which is indicated by 'N' can have a maximum of 32 electrons as 2n2 = 32 and n =4 in this case. Second rule: Shells or orbits are filled one by one in a step-wise manner which means the outer shell cannot be filled until the inner shell is not filled completely or have the maximum number of electrons it can have. For example, only when the 'k' shell has 2 electrons, the next shell 'L' can have 8 electrons. Third rule: The outermost orbit cannot have more than 8 electrons. Let us take an example to understand the rules for electronic distribution; The atomic number of Calcium is 20. So, it has 20 protons, 20 neutrons in the nucleus and 20 electrons around the nucleus. As per the above electronic distribution rules, its first shell will have 2 electrons then we are left with 18 electrons. Now, the second shell can have 8 electrons, or after giving 8 electrons to the second shell, 10 electrons are left. Now, the third shell can accommodate a maximum of 18 electrons. 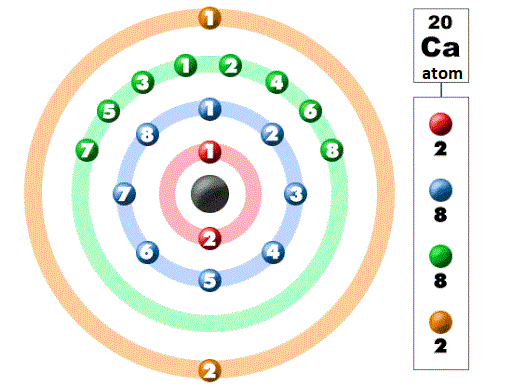
Now, it seems we can put the remaining 10 electrons in the third shell. But, we cannot do this instead we will put 8 electrons in the third shell and the remaining 2 electrons in the fourth shell. The reason for this is a rule that says that there can be a maximum of eight electrons in the outermost shell. In this case, if we allot all of 10 electrons to the third shell then there will be no fourth orbit. It will make the third orbit the outermost orbit with 10 electrons, which will contradict the third rule that says that the outermost orbit can have a maximum of 8 electrons or cannot have 10 electrons. So, as per the third rule, there would be 8 electrons in the third orbit and the remaining 2 electrons will be accommodated in the fourth orbit. Why there are 8 electrons in the third shell of Calcium? Why cannot be the electron arrangement like 2,8,6,4 in this case? The other rule says that unless we fill the inner shells completely we cannot fill the outer shells. So, only when the third orbit gets 8 electrons we can start filling the fourth orbit. What is a subshell?The shell or orbit around the nucleus contains subshells. A subshell is a pathway that is followed by electrons while moving in a shell. It can be of four types, which are s, p, d and f. The first shell (n=1) consists of only one subshell, which is's' and the second shell (n=2) consists of two subshells, which are's' and 'p' and the third shell (n=3) consists of three subshells, 's' 'p' 'd' and the fourth ( n=4) consists of four subshells that include 's' 'p' 'd' and 'f'. Furthermore, each subshell contains one or more orbitals, for example, s contains 1 orbital, p has 2 orbitals, d has 3 orbitals and f has 7 orbitals. Shell is also known as Orbit. So, the orbit is different from the orbital. 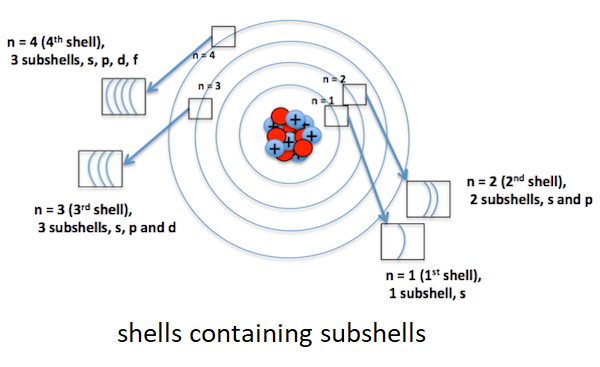
We can say that shell is a collection of subshells or made of subshells that are named s, p, d, and f. The principal quantum number (n) of all subshells remains the same in a shell. For example, n = 1 for the first shell, so, all the subshells present in it will have n = 1. Explanation:Electrons revolve around the nucleus in different shells. Each shell contains subshells with the same principal quantum number (n). For example, the 3s, 3p and 3d subshells belong to the same shell and have the same principal quantum number (n) 3. A large value of n means the shell is far away from the nucleus. Whereas, the small value of n shows the shell is closer to the centre or nucleus. So, electrons in the same shell have the same value of n and are located at the same distance from the nucleus. Just like a shell is a collection of subshells, a subshell is a collection of or consists of orbitals with the same principal quantum number 'n' and angular momentum quantum number 'l'. The subshells are denoted by letters s, p, d, f, g, h and so on. The angular momentum quantum number ('l') for s subshell is equal to 0; for p subshell 'l' = 1; for the'd' subshell 'l' =2. The orbitals' shape is different in different subshells. The electrons of a subshell, which have the same 'l' revolve around the nucleus in nearly the same shape. Each orbital holds two electrons. The electrons of the same orbital have the same principal quantum number, angular momentum quantum number, and magnetic quantum number 'm', it is the 'm' that differentiates different orbitals of a subshell. The orbitals of the same subshell have the same 'l' and 'n' and subshell with the same 'n' value are part of the same shell. 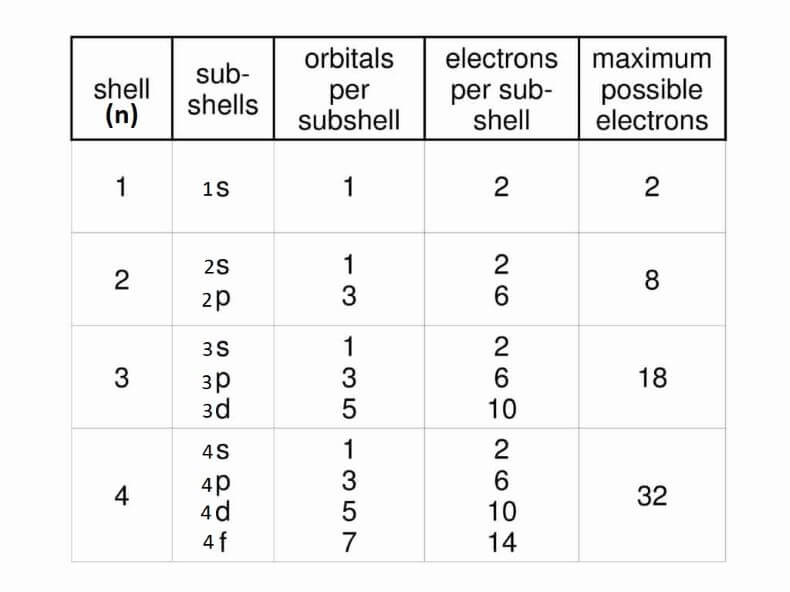
From the above table, it is easy to understand that the first shell has one 1s subshell, and the second shell consists of 2s and 2p subshells. So, subshells are subdivisions of shells and are further divided into or consist of orbitals.
Difference between Shell, Subshell and Orbital
How to write the electronic configuration / Aufbau PrincipleAufbau principle refers to the manner in which electrons should be filled in orbits or valence shells of an atom when it is in the ground state. As per this principle, the electrons are filled into orbitals in the increasing order of orbitals' energy. So, according to the Aufbau principle, the orbits with low energy should be filled first before filling the orbits with high energy. See the image given below, the arrow is showing the order of filling electrons in subshells in the order of increasing energy. Aufbau is a German word whose meaning is 'construct' or 'build-up'. This principle also helps understand the location of electrons and their corresponding energy shells. For example, carbon has 6 electrons so its electronic configuration is 1s2 2s2 2p2. As per the Pauli Exclusion Principle, only two electrons can occupy the same orbital and they must have opposite or antiparallel spins. Besides this, as per Hund's rule, the orbitals should be singly occupied first before pairing the electrons. 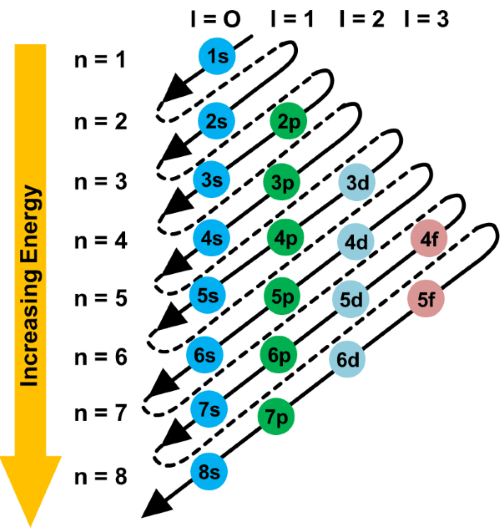
Salient features of the Aufbau Principle
Electronic Configuration according to the Aufbau Principle;Sulphur: Its atomic number is 16, so it has 16 electrons. Now, according to the Aufbau principle, two electrons will occupy the 1s subshell, eight will be present in the 2s and 2p subshell, and the remaining electrons will occupy the 3s and 3p subshells. For example, 1s2 2s2 2p6 3s2 3p4. Nitrogen: It has 7 electrons as its atomic number is 7. Its 7 electrons will occupy 1s, 2s, and 2p orbitals as according to the Aufbau principle, the electrons are filled into 1s, 2s, and 2p orbitals. So, its electronic configuration is written as 1s2 2s2 2p3. Exceptions The electronic configuration of chromium is (Ar) 3d5 4s1 and not (Ar) 3d4 4s2 as it should be according to the Aufbau principle. This exception is connected with various reasons, which include the increase in stability offered by half-filled subshells (all 5 orbitals in d subshell get 1 electron) and the low difference in energy of the 3d and the 4s subshells. 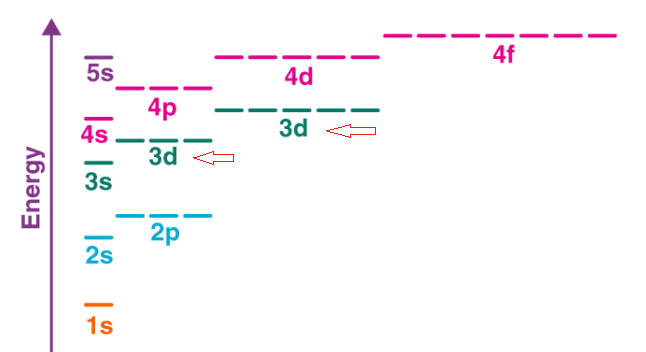
The repulsion between electrons in half-filled subshells is low that tends to increase the stability. Similarly, completely filled subshells also increase the stability of the atom. So, the electronic configuration of some atoms does not obey the Aufbau principle due to the different energy gap between the orbitals. Copper is also an exception to this principle as its electronic configuration is (Ar) 3d10 4s1. This can be due to the stability provided by a completely filled 3d subshell.
Next TopicWhat is valency
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










