Electronic Configuration of First 30 ElementsElectronic ConfigurationThe distribution of electrons within an atom or molecule is referred to as its "electronic configuration," which defines the energy levels and orbitals that the electrons occupy. An element's atomic number, which is equivalent to the number of protons in the atom's nucleus, determines the element's electronic configuration. The amount of electrons in each shell and subshell is typically represented by a sequence of numbers and letters, such as 1s 2s2 2p6, when describing the electronic configuration of an atom. The principal quantum number, which correlates to the electron's energy level or shell, is represented by the first number in the sequence. The angular momentum quantum number determines which letter after the principal quantum number denotes the electron's subshell or orbital. An orbital diagram or electron shell diagram, which shows the arrangement of electrons within the energy levels and orbitals of the atom, can also be used to depict the electronic configuration of an atom. Each orbital is symbolised by a box or circle in an orbital diagram, and each electron is symbolised by an arrow going up or down to denote its spin. An atom's electronic structure plays a significant role in determining many of the element's chemical and physical characteristics. For instance, an atom's reactivity, bonding characteristics, and capacity to take part in chemical reactions are all influenced by the amount and arrangement of its electrons. The amount of energy needed to extract an electron from an atom is known as its ionisation energy, which is also determined by the electronic configuration of the atom. An element's location on the periodic table, which is a list of the elements arranged in increasing order of atomic number, can also be predicted using the element's electronic configuration. The periodic table groups together elements that have comparable electronic configurations and equivalent properties. The Pauli exclusion principle, which asserts that no two electrons in an atom can have the same set of quantum numbers, dictates the electronic configuration of an atom. Accordingly, each electron in an atom must inhabit a distinct energy level and orbital, and each orbital can only accommodate a pair of electrons with the opposite spin. Various spectroscopic methods can be used to directly establish the electronic configuration of an atom. For instance, the electrical configuration of an atom in its ground state can be determined using the emission spectrum of an element, and the energy levels of the electrons in the atom can be determined using the element's absorption spectrum. In conclusion, an atom's electronic configuration is a basic component of its structure and affects a number of its chemical and physical characteristics. An element's atomic number determines its electronic configuration, which can be shown as a series of numbers and symbols, an orbital diagram, or an electron shell diagram. The Pauli exclusion principle, which can be experimentally found using spectroscopic methods, dictates the electronic configuration of an atom. Electronic Configurations are Useful For:
How to Write Electronic ConfigurationShellsBased on the principal quantum number, the greatest number of electrons that can fit in a shell can be calculated (n). The formula for it is 2n2, where n is the shell number. The tables below list the shells, n values, and the overall number of electrons that can fit.
Subshells
Notation
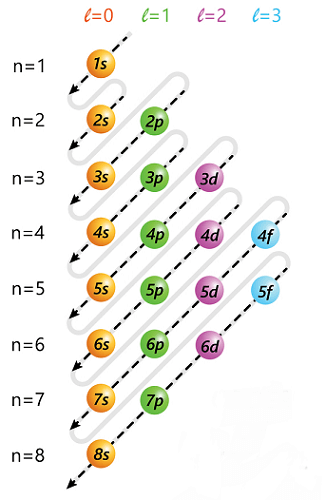
The Aufbau principle, the Pauli exclusion principle, and Hund's rule are used to fill atomic orbitals. These guidelines aid in deciding how the electrons occupy the accessible orbitals. Aufbau Principle:According to the Aufbau principle, electrons occupy orbitals in the direction of increasing energy. This indicates that before filling higher energy orbitals, electrons will first fill the lower energy ones. The periodic table can be used to determine the orbitals' energy levels in order. The labels for the orbitals are a combination of letters and numbers: the letter denotes the orbital shape or subshell (s, p, d, f), and the number denotes the principal quantum number (n), which defines the energy level of the orbital. 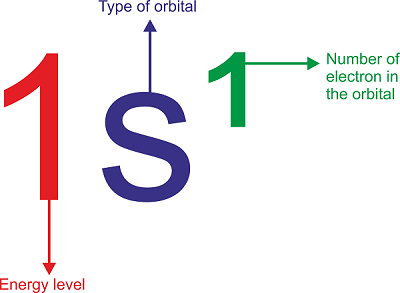
Pauli Exclusion Principle:No two electrons in an atom can have the same collection of four quantum numbers (n, l, ml, and ms), according to the Pauli exclusion principle. The greatest number of electrons that can fit in each orbital is two, and they must have opposite spins. Hund's Rule:According to Hund's rule, electrons will first inhabit separate orbitals with the same spin when filling degenerate orbitals (orbitals with the same energy). Accordingly, electrons in degenerate orbitals will constantly attempt to maximise their total spin. The filling order of atomic orbitals can be established using these principles. The orbitals are filled in the following order:
In conclusion, the Aufbau principle, the Pauli exclusion principle, and Hund's rule all control how atomic orbitals fill up. Each element has a different configuration of electrons as a result of these rules, which help to determine the order in which electrons occupy the available orbitals. Electronic Configuration of the First 30 Elements, in Order of Increasing Atomic Number:
Here are some of the Reasons Why Electronic Configuration is Essential:1. Chemical ReactivityAn atom's chemical reaction is determined by its electronic configuration. The electronic configuration is what causes reactions between elements to result in compounds. How easily an atom can acquire, lose, or share electrons in order to form chemical bonds with other atoms depends on the number and arrangement of electrons in the outermost energy level, known as the valence shell. For instance, in order to achieve a stable configuration, elements with one or two electrons in their outermost shell tend to lose those electrons, whereas elements with five, six, or seven electrons in their outermost shell tend to acquire those electrons. This helps in predicting the kinds of compounds that various elements can create. 2. Bonding PropertiesThe kinds of chemical bonds that can develop between atoms are also determined by their electronic configuration. Covalent bonds typically form between atoms with comparable electronic configurations, whereas ionic bonds typically form between atoms with different configurations. The intensity and stability of the chemical bonds created are also influenced by the electronic configuration. For instance, the four valence electrons in the carbon atom's electronic configuration enable it to form stable covalent bonds with other carbon atoms, which results in the creation of a wide variety of organic compounds. 3. Physical PropertiesThe physical characteristics of an element, such as its melting and boiling points, density, and conductivity, are also influenced by its electronic structure. The number of electrons and how they are arranged in the valence shell decides the strength of the atoms' interactions, which affects how an element behaves physically. For instance, because their free electrons are readily able to move and conduct electricity, metals have high electrical and thermal conductivity. 4. Periodic TrendsThe periodic table is organised using periodic trends because it is founded on the electronic structure of atoms. The regular patterns of variance in the properties of elements across the periodic table are referred to as periodic trends. Changes in the electronic configuration of atoms and their impact on the size, reactivity, and bonding characteristics of elements can be used to understand these trends. To sum up, knowledge of an atom's electronic configuration is necessary to comprehend both its molecular and physical characteristics. It is essential for predicting an element's chemical behaviour and capacity to combine with other elements to create compounds. Understanding electronic configuration also aids in explaining periodic patterns and differences in elemental properties across the periodic table. |
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










