Electrophoresis DefinitionElectrophoresis is a technique that uses an electric field to separate macromolecules in a fluid, or gel based on their charge, binding affinity, and size. Cataphoresis refers to the electrophoresis of positively charged particles (cations), whereas anaphoresis refers to the electrophoresis of negatively charged particles (anions). 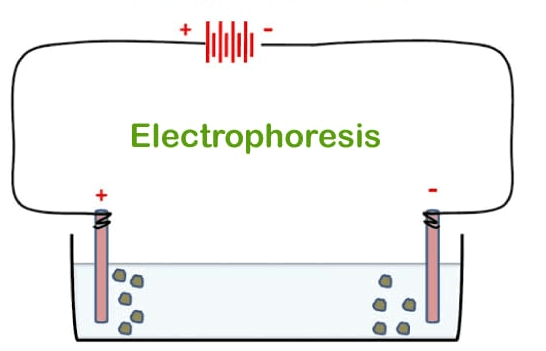
The electrokinetic phenomena of electrophoresis were discovered in 1807 by Russian scientists Peter Ivanovich Strakhov and Ferdinand Frederic Reuss at Moscow University, who discovered that applying a steady electric field caused clay particles distributed in water to move. The presence of a charged contact between the particle surface and the surrounding fluid eventually causes this phenomenon. It serves as the foundation for analytical techniques used in chemistry to separate molecules based on size, charge, or binding affinity. Key Points
What is Electrophoresis?Two basic parameters in electrophoresis affect how rapidly and in which direction a particle can move. Positively charged species are drawn to the positive pole of an electric field, while negatively charged species are drawn to the negative pole. If the field is strong enough, a neutral species can be ionized. Otherwise, it is not usually affected. Where the particle size is another consideration. Smaller ions and molecules can flow significantly faster through a gel or liquid than larger ones. While a charged particle in an electric field is attracted to an opposite charge and other forces will influence how molecules should move. Friction and the electrostatic retardation force slow particle movement through the fluid or gel. The concentration of the gel can be changed in gel electrophoresis to determine the pore size of the gel matrix by affecting mobility. A liquid buffer is also present, which regulates the pH of the environment. 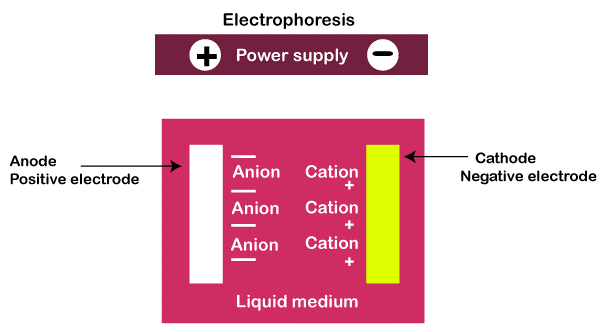
The medium warms up as molecules are drawn through a liquid or gel. This can denature the molecules and change their velocity of movement. The voltage is adjusted to reduce the time required to separate molecules while maintaining a decent separation and keeping the chemical species intact. To compensate for the heat, electrophoresis is sometimes performed in a refrigerator. The processes differ slightly, but we only need an electrical charge source, a support medium, and a buffer solution for electrophoresis. In laboratories, electrophoresis is also used to separate molecules depending on their size, density, and purity. 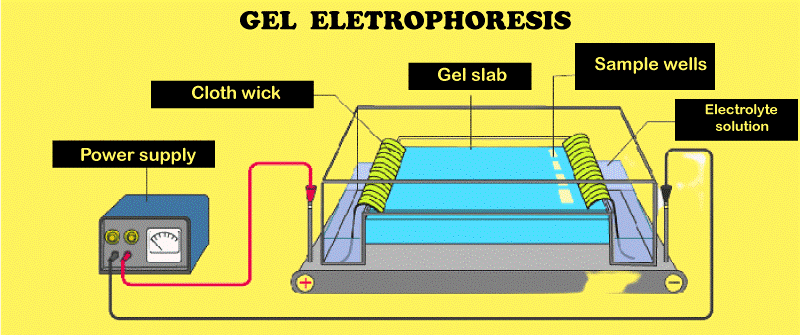
Gel electrophoresis is a technique for separating macromolecules such as DNA, RNA, or protein molecules. In forensics, it is used for -
Clinical Applications of Electrophoresis
History of ElectrophoresisThe history of electrophoresis for molecular separating and chemical analysis began with Arne Tiselius in 1931, while novel separation procedures and chemical analysis techniques that depend on electrophoresis continue to be developed in the 21st century. Tiselius created the "Tiselius apparatus" for movable boundary electrophoresis, published in 1937 in the well-known work "A Novel Apparatus for Electrophoretic Investigation of Colloidal Mixtures" with sponsorship from the Rockefeller Foundation. The approach spread slowly until the 1940s and 1950s when effective zone electrophoresis methods using filter paper or gels as supporting media became accessible. By the 1960s, increasingly sophisticated gel electrophoresis procedures allowed biological molecules to be separated based on minute physical and chemical differences, contributing to the rise of molecular biology. Gel electrophoresis and similar techniques became the basis for various biochemical approaches, such as protein fingerprinting, Southern blot, various blotting procedures, DNA sequencing, and many more. Electrophoresis PrincipleThe existence of charge separation between the surface of a particle and the fluid immediately around the fundamental basis can be referred to as electrophoresis. When an electric field is applied to the resulting charge density, the particle migrates, and the fluid around the particle flows. An electric field is applied to the charged molecules, which are put at one end of the field based on their charge. Depending on the type of charge the molecules carry, they migrate towards the opposite electrodes - either cathode (negative electrode) or anode (positive electrode) (positive electrode). The molecule's size, shape, and charge stay constant during electrophoresis, determining ionic particle mobility. 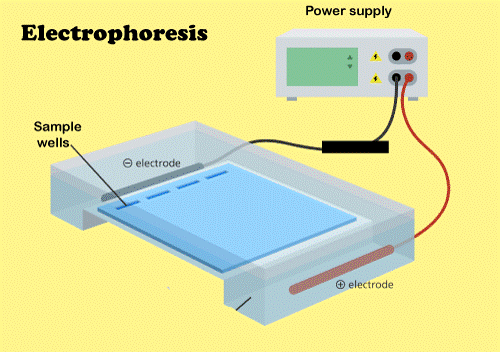
In acidic conditions, an ampholyte acts as a positively charged particle or ion traveling toward the cathode. In an alkaline environment acts as a negatively charged particle or ion and travels toward the anode. The rate of ion migration in a supporting medium under the influence of an electric field is determined by the following factors:
Electrophoresis TypesElectrophoresis refers to a group of similar analytical procedures. Here are several as follows Affinity ElectrophoresisAffinity electrophoresis is a form of electrophoresis in which particles are separated depending on their complex formation or bio-specific interaction. Slab Gel ElectrophoresisSlab gel electrophoresis is a common method incorporating a rectangular gel of any thickness. Disc ElectrophoresisDiscontinuities in the electrophoretic matrix are created by polyacrylamide or starch gel layers that vary in composition and pore size. Isoelectric focusing electrophoresis (IEF) separates air substances (such as proteins) with high precision in a medium with a steady pH gradient. Isotachophoresis(ITP)It separates smaller ionic materials into adjacent zones that do not overlap and have the same migration rate. In ITP, a sample comprising various charged molecules is pumped into a tube containing a buffer system made up of many zones of electrolytes with varying conductivity. The tube is then subjected to an electric field, which causes the charge molecules to move toward their appropriate zones depending on their electrophoretic mobilities. Capillary ElectrophoresisA capillary electrophoresis is a form of electrophoresis used to separate ions based on their atomic radius, charge, and viscosity. As the name implies, this method is often conducted in a glass tube. It produces speedy results with high-resolution separation. Gel ElectrophoresisGel electrophoresis is a type of electrophoresis in which molecules are separated by moving across a porous gel while being influenced by an electrical field. Agarose and polyacrylamide are the two most used gel components. Nucleic acids (DNA and RNA), nucleic acid fragments, and proteins are separated using gel electrophoresis. Paper ElectrophoresisPaper electrophoresis is a simple and inexpensive analytical technique for separating and studying charged molecules such as proteins, amino acids, as well as nucleic acids. It involves presenting an electric field to a small amount of the sample and allowing the charged molecules to travel through the paper dependent on their charge and size. ImmunoelectrophoresisImmunoelectrophoresis is the general name given to a variety of electrophoretic procedures used to describe and segregate proteins based on their reactivity to antibodies. Zone ElectrophoresisZE, or Zone electrophoresis, is a method for analyzing nucleic acids, biopolymers, and proteins. This electrophoretic separation method involves transporting several species in a buffer solution while subjected to an electric current. These species will divide into well-resolved and variable peaks due to differences in mobility. Pulsed-field Gel ElectrophoresisPulsed-field electrophoresis is a method to separate macromolecules, such as DNA, by periodically altering the direction of the electric field applied to a gel matrix. Because standard gel electrophoresis is incapable of efficiently separating very big molecules that tend to migrate together where the electric field is different. Shifting the direction of the electric field provides the molecules with new paths to go through the gel. The voltage is often alternated between three directions in which one parallel is to the gel's axis and two is at 60 degrees to either side. Although it takes longer than ordinary gel electrophoresis, it is more effective at isolating big bits of DNA. Isoelectric FocusingIsoelectric focusing (IEF or electrofocusing) is an electrophoresis that separates molecules based on their isoelectric points. IEF is most used on proteins since their electrical charge is pH dependent. The ConclusionElectrophoresis is an electrokinetic phenomenon that involves an electric field, a supporting medium, and a buffer solution. It has a wide range of forensic and clinical uses in everyday life. Electrophoresis has come a long way since its invention in Russia in 1807, from basically rectangular gels to higher resolution techniques. Generally, electrophoresis is a technique for separating biomolecules or charged particles of interest based on their mobility in a particular electric field. Gel electrophoresis allows scientists to see the sizes of DNA segments and aids in DNA sequencing.
Next TopicEmphysema Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










