Fatty AcidsFatty acids (FAs) are the main components of some types of lipids (fats) like phospholipids and triglycerides. They are made of carbon, hydrogen and oxygen atoms and are arranged as a linear chain of carbon atoms bonded with hydrogen atoms and a carboxyl group at one of the ends. Generally, it has an even number of carbon atoms with a carboxyl group at the end. This end acts as a reaction part of the fatty acid molecule and thus participates in chemical reactions to make lipids, which store energy. A fatty acid can have 2 to 30 carbon atoms. However, the most important and commonly found fatty acids have 12 to 22 carbon atoms and are naturally found in animal and plant fats. Basic structure of fatty acids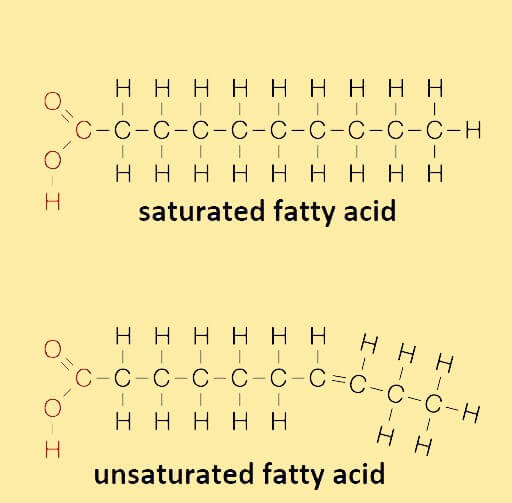
A molecule of a fatty acid comprises a chain of carbon atoms bonded with hydrogen atoms and at one end of this chain, a carboxyl group is present, whereas, at another end a methyl group is present. The carboxyl group is bonded with the hydroxyl groups (-OH) on a glycerol molecule in a process called dehydration synthesis. When this bond is formed, energy is stored and water is released. The methyl group is called the omega (ω) and the carbon located beside the carboxyl group is called the alpha "α" carbon and the carbon atom beside the alpha "α" carbon is known as the beta "β" carbon. Further, the fatty acid molecule also has two distinct regions: a less reactive or inert long hydrophobic hydrocarbon chain and a carboxyl group, which is reactive and hydrophilic (water-loving). The cell membrane also has fatty acids, wherein, virtually all fatty acids are connected with each other through covalent bonds formed via their carboxylic acid groups. The fatty acids differ from each other in terms of the number of carbon atoms present in them. However, the number of carbon atoms is generally even in number. The carbon chain of a fatty acid can be as short as made of only two carbon atoms or may be as long as it needs to be depending on the functions it performs. Classification of fatty acidsBased on the degree of saturation or unsaturation, they are of the following three types: Saturated fatty acids: Fatty acids that don't contain double bonds are called saturated fatty acids. They have more hydrogen atoms than unsaturated fatty acids. They have a linear structure and originate from animal sources and are generally solid at room temperature. If they have one or more double bonds they are called unsaturated fatty acids, which are further of different types as described below: Monounsaturated: The fatty acids that contain one double bond are called monounsaturated fatty acids. Polyunsaturated: The fatty acids that contain multiple (more than one) double bonds are called polyunsaturated fatty acids. Unsaturated fatty acids occur in two specific structural configurations: cis and trans isomers as described below: Cis: In this structural configuration, the hydrogen atoms attached to the carbon with a double bond are located on the same side. Trans: In this configuration, the hydrogen atoms bonded to the carbon with a double bond are located on the opposite sides. Trans fatty acids are not commonly found in nature and are generally produced by hydrogenation, an industrial process. Despite being unsaturated, trans fatty acids have a linear structure and are solid at room temperature. Further, based on the human body's ability to synthesize fatty acids, they can be classified into two types: essential fatty acids and nonessential fatty acids:
Despite having the above difference, these two types of fatty acids have some similarities: both are made of long hydrocarbon chains with a carboxylic group at one end and both play a vital role in the synthesis of triglycerides. Applications of fatty acids
Next TopicAzelaic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










