Hydrocarbons DefinitionDefinitionThe definition of "Hydrocarbon," which denotes only substances made of carbon and hydrogen, is self-explanatory. Hydrocarbons are essential to our everyday existence. The terms used to describe the fuels, like "LPG" and "CNG," that one must be aware of. Liquid petroleum gas is called LPG, while compressed natural gas is called CNG. These days, the word "LNG" (liquified natural gas) is also commonly used. This can be made by liquifying natural gas and is also a fuel. 
Fractional distillation of petroleum beneath the earth's surface produces gasoline, diesel fuel, and kerosene oil. By distilling coal in a harmful manner, coal gas is produced. As hydrocarbon wells are drilled, natural gas is discovered in the upper strata. Natural gas that has been compacted is referred to as compressed gas. The least polluting fuel for household use is LPG. Although it is also used as a household fuel, kerosene oil pollutes. Fuels like gasoline, petroleum, and compressed natural gas are necessary for automobiles. Automobiles running on gasoline and CNG emit less smog. These fuels all comprise a mixture of hydrocarbons, which are energy-producing substances. The production of plastics like polystyrene, polypropene, and polythene also uses hydrocarbons. Solvents for pigments are made from higher hydrocarbons. Additionally, they serve as the raw materials for producing numerous colours and medications. You can see how important hydrocarbons are to your everyday life from this. You will learn more about hydrocarbons in this article. Types of HydrocarbonsThe simplest organic molecules, aside from alcohols, aldehydes, and carboxylic acids, are hydrocarbons, which fall into one of the following categories: 1. Saturated Hydrocarbons"Saturated" hydrocarbons only contain one link between the carbon atoms. They are the compounds that are the simplest to comprehend. Because each carbon atom is connected to as many hydrogen atoms as possible, they are called saturated. In other words, the carbon atoms have excessive hydrogen in them. Saturated hydrocarbons are hydrocarbons in which all carbon atoms are bonded to four other atoms. Almost all saturated compounds are referred to as alkanes. Alkanes' shapes The structural formulas of hydrocarbon compounds, which depict how the atoms are organized, are frequently used to illustrate shape. This is so because the formula reveals the atoms' arrangement while the molecules can have various forms. Chains, chains with branches, and rings can form when hydrocarbons combine. In a straight-chain molecule, all carbon atoms are arranged in a straight path, much like the cars on a train. The carbon atoms hold the molecules together. While in a ring structure, each ring typically contains only five or six carbon atoms. Rings can, however, combine to form bigger molecules. Cyclic compounds often have greater melting and boiling temperatures than straight-chain and branched-chain molecules. Alkenes Unsaturated hydrocarbons (compounds comprised exclusively of carbon and hydrogen) with at least one double bond between carbon atoms are called alkenes. Alkenes can also be referred to as olefins. An alkene is more reactive than an alkane due to its double bond. Alkenes and ethene, in particular, are crucial to the chemical industry. Crude oil does not contain them in significant quantities. Instead, the alkanes are cracked to create them. All hydrocarbons, including alkenes, release carbon dioxide and water when they burn in the atmosphere. Ethene is a poor fuel because it interacts violently with oxygen. Alkynes An unsaturated hydrocarbon with at least one triple bond between the carbon atoms is an alkyne in organic chemistry. Alkynes are typically hydrophobic, just like other hydrocarbons. The most common name for ethyne is "acetylene," which is ridiculous. It is the most straightforward alkyne. Each carbon atom can only link to one hydrogen atom due to the triple bond that connects its two carbon atoms. Saturated Hydrocarbon Types A saturated hydrocarbon can belong to one of the following groups depending on whether its structure is straight, branched, or ring-shaped: Alkanes: Alkanes are organic substances that solely include carbon and hydrogen atoms and are linked by a single bond; they do not contain any additional functional groups. They can be categorized into linear straight-chain alkanes, branched alkanes, and cycloalkanes. They have the general formula Cn H2n+2. Alkanes are saturated hydrocarbons as well. The simplest and least reactive hydrocarbons are alkanes. They don't interact with other things because they contain carbon and hydrogen. Cycloalkanes: Alkanes that contain one or more carbon rings are known as cycloalkanes. They are physically identical to alkanes, but because they have stronger London forces, they have higher melting and boiling temperatures and densities. What are Saturated Hydrocarbons Used For?
2. Unsaturated hydrocarbonsUnsaturated hydrocarbons are organic molecules that only include carbon and hydrogen atoms and have two or three bonds between the two carbon atoms next to each other. For instance, Propylene and CH3CH2CH=CH2 (n-Butylene). Unsaturated and saturated hydrocarbons have a lot in common regarding their physical characteristics. Except for aromatic hydrocarbons, these types of hydrocarbons are very reactive and frequently engage in additional reactions with various compounds, including alcohols, hydrogen halides, and elemental halogens. Unsaturated hydrocarbon types
Unsaturated hydrocarbon uses The following is a list of some applications for various unsaturated hydrocarbon molecules.
Saturated Versus Unsaturated Hydrocarbon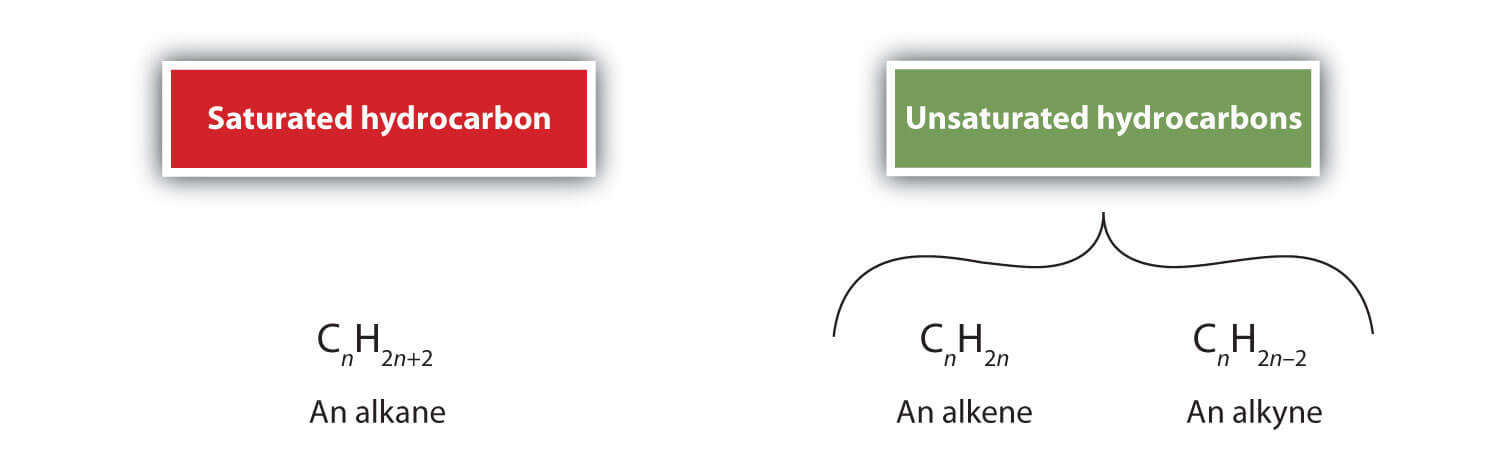
Saturated hydrocarbons only have single covalent bonds between carbon atoms, but unsaturated hydrocarbons have at least one double or triple covalent link in the main chain. This is the major distinction between saturated and unsaturated hydrocarbons. Saturated and unsaturated hydrocarbons have various characteristics because of their differing compositions. Hydrocarbons Examples Methane and propane are examples of gaseous hydrocarbons; hexane and benzene are examples of liquid hydrocarbons; paraffin wax and naphthalene are examples of low melting point solids or waxes. The saturated members of the hydrocarbon family are especially notorious for being inert. Others, however, will have one of the three main types of reactions described below: 1. Substitution Reaction The term "substitution reaction" refers to a reaction in which another group replaces one chemical substance's functional group or when another molecule replaces one atom or molecule of a compound. Types of Substitution Reactions There are two types of substitution reactions: Nucleophilic reactions and electrophilic reactions. The primary distinction between these two reactions is the type of atom joined to the initial molecule. Atoms are referred to as electron-rich species in nucleophilic reactions and electron-deficient species in electrophilic reactions. 1. The Nucleophilic Substitution Reaction (a) What are nucleophiles? The species tightly bonded to a positive charge region are known as nucleophiles. They can take the shape of an ion or a molecule. They are referred to as being completely charged or having negative ions on a molecule. Water, hydroxide ions, ammonia, cyanide ions, and water molecules are typical examples of nucleophiles. (b) Nucleophilic substitution reaction mechanism Here, two nucleophilic substitution reaction processes are discussed. S refers to chemical substitution, N stands for nucleophilicity, and the number denotes the kinetic order of a reaction. These reactions are the SN1 reaction and the SN2 reaction. SN2 Reaction - SN2 Reaction Mechanism The removal of the leaving group and the addition of the nucleophile occur concurrently in this reaction. Where the central carbon atom may easily contact the nucleophile, SN2 occurs. Several different factors influence the rate of SN2 reactions. They are as follows:
SN1 Reaction - SN1 Reaction Mechanism Several variables also impact the SN1 reaction. Here is a handful that is covered:
2. Electrophilic Substitution Reactions (a) What are electrophiles? The electrophiles are involved in the electrophilic substitution process. Electrophiles are substances that contribute two electrons to create a covalent bond. The majority of electrophilic reactions involve aromatic molecules. These compounds have a surplus of electrons that can be distributed among other reactants. (b) What exactly is the Electrophilic substitution reaction? The chemical reactions known as electrophilic substitution reactions are those in which the electrophile replaces the functional group in a molecule but leaves the hydrogen atom alone. The hydronium ion (H3O+), halides of hydrogen-like HCl, HBr, and HI, sulfur trioxide (SO3), the nitronium ion (NO2+), and others are examples of species of electrophiles. (c) Electrophilic substitution reaction types Here, we describe two different kinds of electrophilic substitution processes. There are two types of electrophilic substitution reactions:
An atom linked to the aromatic ring, primarily made of hydrogen, is substituted by an electrophile in this sort of electrophilic substitution. Aromatic halogenation, alkylating Friedel-Crafts processes, aromatic nitration, aromatic sulfonation, and aromatic acylation is the reactions. Alkylation and acylation are also included.
An electrophile dislocates one functional group in this kind of electrophilic substitution process. What is the definition of an Addition Reaction? The simplest definition of an addition reaction in organic chemistry is a chemical process in which two or more reactants combine to create a bigger single product. Explanation of Addition Reaction However, because a double or triple bond is typically broken to create the necessary single bonds, only chemical compounds with multiple bond characteristics can undergo an addition reaction. A decomposition reaction is when a chemical breaks down into one or more elements or compounds. In essence, an addition reaction is the opposite of a decomposition reaction. Consider the hydrochlorination of propane (an alkene), which has the following: CH3CH = CH2 + HCl → CH3C+HCH3 + Cl− → CH3CHClCH3 Use of hydrocarbons in real life1. Hydrocarbons make up plastics Plastic is crucial to our daily life. Every day, we make use of it in some way. Plastic cups, shopping bags, meal trays, containers, and bottles are just a few examples. Hydrocarbons like polyethene, polypropylene, and polystyrene are used to make plastics. 2. Alkanes in Everyday Life We encounter so many different alkanes daily. Electricity is produced via the utilization of methane. It is often made from landfill-sourced household and commercial garbage, which microbes break down to release methane gas. Then, this is put to use to make power. 3. Combustion When hydrocarbons are burned, heat sources and electrical energy are generated. It can be utilized as natural gas or petroleum-based house heater. Hydrocarbons burn to produce heat, carbon dioxide, and steam when oxygen is present. Hexane and other alkanes are utilized in glues for shoes and leather goods, while butane is used in aerosol cans. Gasoline, which is used as a fuel, contains heptane and other alkanes, including octane and decane.
Next TopicHydrosphere Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










