Isotopes and IsobarsIsotopes refer to the different atoms or forms of an element with the same atomic number but different atomic masses. So, they contain the same number of protons and electrons but differ in the number of neutrons. For example, Carbon -14, Carbon-13, and Carbon-12 are isotopes of carbon as C-14 has 8 neutrons, C-13 has 7 neutrons and C-12 has 6 neutrons, whereas, all of them have the same number of protons that is 6 and the same number of electrons that is 6. 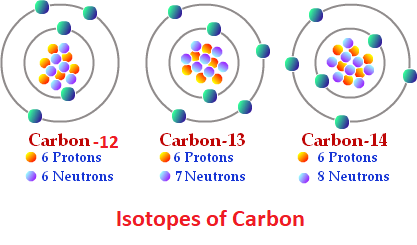
The term 'isotope' was given by Frederick Soddy in 1913 as suggested by Margaret Georgina Todd. It is originated from two Greek words 'isos' and 'topos' which means 'the same place'. This term was chosen for isotopes as they occupy the same place in the periodic table although they differ in their atomic masses. Each box in the periodic table that is occupied by an element actually represents a group of isotopes or atoms with the same chemical name and chemical properties but different atomic masses. So, every element has multiple isotopes one of which is generally more abundant in nature than its other isotopes and has the same number of protons and neutrons such as C-12 is the most abundant isotope of carbon with 6 protons and 6 neutrons. However, the most abundant isotope of hydrogen (protium) is an exception as it has one proton but does not contain any neutron. Besides this, isotopes have nearly the same chemical properties. However, there are some exceptions such as isotopes of hydrogen as the number of neutrons significantly affect the size of the hydrogen atom nucleus. The physical properties of isotopes are not the same as physical properties depend on atomic masses which is different for isotopes. So, the isotopes generally differ in their melting or boiling points, densities, freezing points, etc. This difference in physical properties helps separate isotopes of an element through fractional distillation and diffusion. Isotope Notation I how isotopes are represented? Isotopes are mainly represented in two different ways, which are as follows
What are parent and daughter isotopes?When a radioisotope undergoes radioactive decay, the initial isotope becomes different from the resultant isotope. Here, the initial isotope, when an isotope starts to decay, is known as the parent isotope, whereas, the resulting isotope is called the daughter isotope. For example, when the U-238 isotope produces Th-234 after decay, so, the uranium atom is called the parent isotope and the thorium atom is called the daughter isotope. Types of isotopesAll isotopes are not radioactive. Based on this fact, they are mainly of two types that include stable isotopes and radioactive isotopes. Stable isotopes: As the name suggests, they have stable nuclei and do not undergo decay or they decay at a very slow rate. They have a stable combination of neutrons and protons. These isotopes are not harmful to living things like radioactive isotopes. So, stable isotopes do not undergo radioactive decay. However, if they undergo, they decay at a very slow rate and due to this reason, they are still considered stable isotopes. For example, Bismuth-209 is a stable radioactive isotope as its half-life is 1.9 x 1019 years that is more than the expected age of the universe so even though it decays it is considered a stable radioactive isotope. So, isotopes with extremely long half-lives referred to as stable isotopes or stable nuclides. For example, carbon-12, oxygen-16, oxygen-17, etc. Radioactive isotopes: They are not stable and always undergo decay as they have an unstable combination of neutrons and protons. So, isotopes that have unstable nuclei tend to undergo radioactive decay and thus are radioactive in nature and known as radioisotopes or radionuclides. For example, C-14, tritium (hydrogen-3), uranium-235, uranium-238 are some of the radioactive isotopes. Furthermore, there are also primordial nuclides, which are present since the solar system's formation. It is believed that out of 339 isotopes that are found naturally, 286 isotopes are considered primordial isotopes. How to find out the number of neutrons in an isotope?The atomic mass of an element is the sum of protons and neutrons present in it. So, to calculate the total number of neutrons in an isotope or an atom, we can subtract the total number of protons from atomic mass. The atomic number is equal to the total number of neutrons, so we can say that we can subtract the atomic number of an element from its mass number to determine the neutrons present in it. For example, the atomic mass or mass number of C-12 is 12 and its atomic number is 6, so, it will have 12-6 = 6 neutrons. General characteristics of isotopes
IsobarsIsobars are the atoms of different elements whose atomic numbers are different but mass numbers are the same. As their atomic number is different, they have a different number of protons and electrons as we know that atomic number is equal to the number of protons and electrons. Now, they have the same mass number which is made of protons and neutrons, now protons are different, so neutrons are also different in them if they have the same number of neutrons their mass number could not be the same. 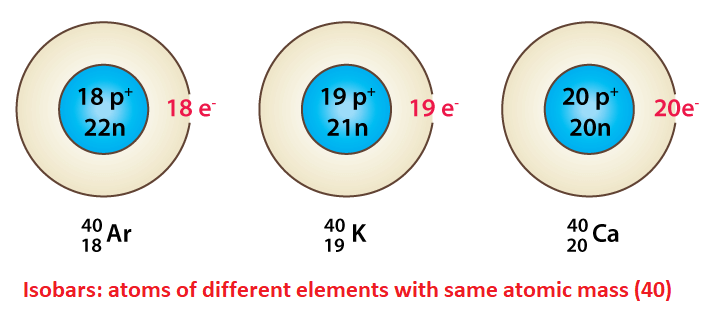
For example, atoms of Argon and Calcium (40 18Ar and 40 20Ca ) are isobars. They have the same atomic mass, which is 40, but a different number of protons and electrons 18 and 20 and a different number of neutrons 22 (40-18) and 20 (40-20), respectively. Alfred Walter Stewart proposed the term isobars in 1918. It is taken from the Greek words 'isos' and 'baros' which mean equal and weight respectively. Difference between isotopes and isobars in tabular form
Let us see some solved numerical to understand isotopes and isobars. 1) Which of the following pairs are isotopes and which are isobars?i) 23592U and 23892U ii) 4019K and 4020Ca iii) 31H and 32He iv) 168Y and 188Y Solution (i): In the first case both the atoms belong to the same element. So, they will have the same atomic number which is 92 as given in the sum. If we look at their atomic masses, they differ in their atomic masses. So, we can say that they represent the isotopes of Uranium. Solution (ii): In this case, we have one atom of potassium (K) and one atom of calcium (Ca), which means we have two atoms that belong to two different elements. Now, if we look at their atomic numbers, it is different (19 and 20), however, if we look at atomic masses, both have the same atomic mass (40 and 40). So, we can say that they are isobars. Solution (iii): In this case, we have atoms of different elements, so their atomic number is different (1 and 2). But, they have the same atomic mass or mass number which is 3. So, these two atoms are isobars. Solution (iv): Here, we can see both the atoms belong to the same element Y. Now, their atomic number is given the same but their mass number or atomic mass is different. So, they are termed isotopes. So, in the above four cases, we have two isotopes (i and iv) and two isobars (ii and iii). Now, let's calculate the difference in the number of neutrons in the pairs of isotopes in the case of 23592U and 23892U . In U-235, we have an atomic number 92 and the atomic mass is 235. As the atomic number is 92, so, the number of protons will be equal to 92. Now, atomic mass (235) is equal to the sum of protons and neutrons. So, we have 235 = neutrons + 92 So, neutrons = 235 - 92 = 143 Now, in the second isotope of Uranium, the atomic number is 92 and the atomic mass is 238. So, in this case neutrons = 238 - 92 = 146 Now, the difference in the number of neutrons in the given isotopes or atoms of uranium is 146 - 143 = 3 neutrons.
Next TopicEvaporation
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










