Latent Heat DefinitionDue to the difference in the temperature of the two materials, heat energy can be transmitted from one material to another. It is a kind of kinetic energy linked to the irregular movement of a substance's constituent particles. The letter Q frequently represents heat energy, expressed in joules. Several processes, including friction, combustion, and nuclear reactions, can produce it. The amount of heat energy exchanged between two bodies relies on their respective temperatures as well as the thermal conductivity of the material. A key idea in thermodynamics is that heat energy is vital to numerous industrial and natural processes. Let us talk about latent heat. "Latent Heat" refers to the energy a substance acquires or emits during a phase change, which is crucial in thermodynamics. This heat is referred to as "latent" because it does not cause a change in the substance's temperature but rather a change in its internal energy. To understand latent heat, defining what a phase change means is necessary. A phase change occurs when a substance transitions from one state of matter to another, such as converting from the solid state of matter to the liquid state of matter or a liquid to a gaseous state of matter. It is important to note that during a phase change, the temperature of the body/material stays non-changed even though energy is being added or removed from the system. This energy is what is referred to as latent heat. 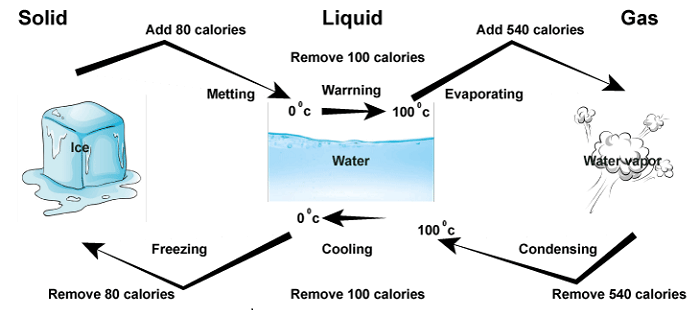
There are two types of latent heat: latent heat of fusion and vaporization. Latent Heat of Fusion is defined as the amount of energy required to melt (fuse) a substance (phase change included here is from solid to liquid). In contrast, latent vaporization heat is the energy required to change the liquid into the gaseous state. Both types of latent heat represent the energy required to break the intermolecular forces of attraction that hold the particles of a substance together. The intermolecular force of attraction is the reason for matter's different states and shapes. For example, let us consider the process of melting ice. When ice is heated, its temperature will rise until it reaches 0°C (32°F). At that point, it will begin to melt. During this phase change, the temperature of the ice-water mixture remains constant even though heat is being added to the system. This is because mostly all heat energy is utilized to break the bonds between the water molecules in the ice rather than increase the system's temperature. The latent heat required to melt ice is the latent heat of fusion. This value is specific to water and equals 334 joules per gram. In another language, 334 joules of energy are needed to melt one gram of ice at 0°C. Similarly, when water is heated to its boiling point, it undergoes a phase change from a liquid to a gas. During water heating, the water's temperature tops at 100°C (212°F) even though more heat is being supplied to the system. This energy breaks the intermolecular bonds between water molecules and turns the liquid into water vapour. The energy required to do this is known as the latent heat of vaporization, equal to 2,260 joules per gram of water. Latent heat plays a major role in many natural phenomena. For example, the release of latent heat during the process of condensation is what drives thunderstorms and hurricanes. When moist air rises and gets cool, it attains its dew point and condenses, excreting the latent heat of vaporization. The air may ascend even more quickly due to the added heat, resulting in thunderstorms or hurricanes. 1. Latent Heat of Fusion (LHF)The heat energy required to transform a substance from a solid state to a liquid state at its melting point without causing a temperature change is the latent heat of fusion. During the fusion process (melting), the solid matter receives heat energy from its surroundings, which is used to overcome the intermolecular forces between the molecules in the solid and convert it into a liquid state. This absorbed heat energy is known as the latent heat of fusion. It is symbolized by Lf. The latent heat of fusion is an important concept in many areas of science and engineering, including material science, thermal engineering, and meteorology. For example, material science is used to understand the behaviour of materials during phase transitions, such as the formation of crystals from a melt.
The value of the latent heat of fusion is specific to each substance and is expressed in units of energy per unit mass or joules (J). For example, the latent heat of the fusion of water is 334 joules per gram. It says the energy required for fusing 1 gram of ice at 273k is 334 joules. In conclusion, the latent heat of fusion is an important concept used in many applications, including material science, thermal engineering, and meteorology, and its value is specific to each substance. 2. Latent Heat of Vaporization (LHV)Latent heat of vaporization is the heat energy needed to alter the state of a body from the liquid state to the gaseous state at its boiling point without any temperature change. During vaporization, the liquid absorbs heat energy from its surroundings, which is used to overcome the intermolecular forces between the molecules in the liquid and convert it into a gaseous state. This absorbed heat energy is known as the latent heat of vaporization. It is symbolized by Lv. The latent heat of vaporization is an important concept in many areas of science and engineering, including thermal engineering, material science, and meteorology. For example, thermal engineering is used to understand the behaviour of cooling systems that rely on phase changes, such as refrigeration and air conditioning units. In these systems, a refrigerant absorbs heat from the surroundings and undergoes a phase change from a liquid to a gas, which allows it to cool the surrounding environment. When the refrigerant is pressurized, it emits the absorbed heat and condenses back into the liquid state, releasing its latent heat of vaporization. The value of the latent heat of vaporization is specific to each substance and is expressed in units of energy per unit mass. The latent heat of the vaporization of water is 2,260 joules per gram. It says the energy required to vaporize 1 gram of water at 373k is 2260 joules. In conclusion, the latent heat of vaporization is an important concept in science and engineering. This concept is used in many applications, including thermal engineering, material science, and meteorology, and its value is specific to each substance. Daily Life Examples Based on Latent HeatWe Feel Cool While Sweating: The phenomenon behind this is that evaporation causes cooling. In a hot season, our body becomes hot, and sweat glands release sweat outside the body. The sweat present takes heat energy from our body to change its state from liquid to gas. This leads to decreased body temperature and provides a cooling effect to the body. DifferenceThe latent heat of fusion and the latent heat of vaporization are both types of latent heat. However, they are different regarding the specific phase change they describe. Here is a tabular difference between the two:
Overall, both the latent heat of fusion and the latent heat of vaporization are important properties to consider when studying phase changes in materials. ApplicationsLatent heat has many applications in different fields, such as engineering, physics, chemistry, and meteorology. Here are some of the applications of latent heat:
Overall, latent heat is an essential concept with many practical applications in different fields, and its properties are critical to understanding the efficient design of various systems and processes. NumericalHere are some examples that demonstrate the concept of latent heat: The general formula to calculate latent heat is given: Q = M * L (Joules) Where Q is the amount of the total heat absorbed or released. L is the specific latent heat (mostly given) And M is the total mass of the substance. 1. Melting of IceLet us say you have a 1-kg block of ice at -10°C. If you want to melt the ice to liquid water at 0°C, you must supply it with the latent heat of fusion (Lf) to break the intermolecular bonds between the ice molecules. The amount of latent heat required is given by: Lf = 334 kJ/kg (for water) So, the total amount of latent heat required to melt the 1-kg block of ice is: Q = m x Lf = 1 kg x 334 kJ/kg = 334 kJ Therefore, you need to supply 334 kJ of latent heat to the block of ice to melt it completely. Boiling of WaterLet us say you have a pot of water on a stove at 100°C and want to boil the water to steam at 100°C. To do this, you must supply it with the latent heat of vaporization (Lv) to break the intermolecular bonds between the liquid water molecules. The amount of latent heat required is given by: Lv = 2260 kJ/kg (for water) So, the total amount of latent heat required to boil 1 kg of water is: Q = m x Lv = 1 kg x 2260 kJ/kg = 2260 kJ Therefore, you need to supply 2260 kJ of latent heat to the pot of water to convert it into steam completely. Cooling Effect of EvaporationLet us say you have a water container at room temperature (25°C). If you spray some water onto your skin and blow air onto it, you will feel a cooling sensation. This is due to the latent heat of vaporization, where the water absorbs heat from your skin to evaporate and cool it down. The amount of latent heat absorbed by the water is given by: Q = m x Lv = 1 g x 2260 kJ/kg = 2.26 kJ Therefore, 1 gram of water can absorb 2.26 kJ of latent heat during evaporation, which causes a cooling effect. These examples demonstrate the practical applications of latent heat and how it affects various phase changes. ConclusionLatent heat is a critical concept in thermodynamics that describes the energy absorbed or released by a substance during a phase change. The two types of latent heat, latent heat of fusion and latent heat of vaporization, represent the energy required to break intermolecular bonds between particles during melting and vaporization. Understanding latent heat is important for various applications, from weather forecasting to material science.
Next TopicMidwife Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










