Law of Definite Proportions DefinitionThe law of definite proportions states that each component of a chemical compound must be present in a precise and consistent amount (by mass) for the compound to exist. The law of definite proportion, also referred to as Proust's law is a well-known tenet of chemistry. Any specimen of pure water, for example, contains about 8/9 of its mass made up of oxygen, and 1/9 of it is hydrogen. This is an example of how the mass of 2 elements in a compound is always proportionate to one another. The law of definite proportions serves as stoichiometry's foundation together with the law of numerous proportions. 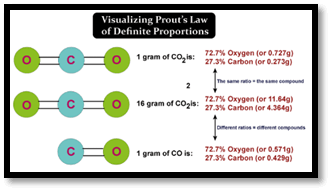
HistoryJoseph Proust first stated the concept of constant proportion in 1797 in Segovia, Spain. 'Antoine Lavoisier', a French nobleman and scientist who focused on combustion, and the English clergyman & chemist Joseph Priestley were the first to make this remark. "I'll sum up by drawing conclusions from these experiments that support the theory I put up at the beginning of this memoir, namely that iron, like many other metals, is subject to the natural law that governs every real combination, which states that it combines with two constant amounts of oxygen. This makes it similar to practically every known combustible, including tin, mercury, lead, and so on." The law of definite proportions may appear clear to a modern scientist because it is included in the basic definition of chemical compounds. However, the legislation was originally at the end of the 18th century since the idea of a chemical compound hadn't yet been properly defined. Claude Louis Berthollet, a fellow Frenchman who studied under Proust, and other scientists contested it when it was first put forth, claiming that the components may combine in any ratio. The fact that this dispute even existed at the time shows that the line between pure chemical substances and mixtures was not yet well established. The atomic theory, promoted by John Dalton beginning in 1803, asserted that everything is made of discrete atoms, that there is one type of atom with each component, & that compounds are composed of combinations of different types of atoms in specified proportions. This theory contributed to and provided a theoretical underpinning for the law of definite proportions. 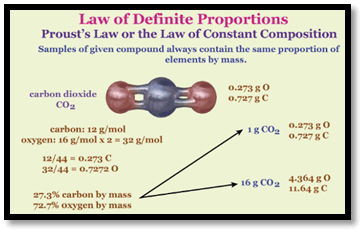
The English chemist William Prout's notion, which claimed that the hydrogen atom had been the basic atomic unit, was a related early theory. The whole number rule-a generalization that atomic masses were multiples of the mass of hydrogen-was developed from this theory. The theory was later disproved in the 1820s and 1830s as a result of more accurate measurements of atomic mass, particularly those made by Jöns Jacob Berzelius. These measurements specifically showed that chlorine had an atomic mass of 35.45, which was inconsistent with the idea. Since the 1920s, isotopes have been used to explain this mismatch. Any isotope's atomic mass comes very close to obeying the whole number rule, and the mass defect brought on by varying binding energies is much smaller. Non-stoichiometric substances and isotopesThe law of definite proportions isn't always true, while immensely helpful in developing modern chemistry. It is possible for non-stoichiometric substances' elemental compositions to change between samples. Such compounds abide by the multiple proportions law. A perfect example is the 'iron oxide wüstite', with a total iron content of 0.83 to 0.95 per oxygen atom and oxygen content somewhere between 23% & 25% by mass. However, due to crystallographic vacancies, the actual formula is closer to Fe0.95O than FeO. Proust's measurements weren't generally accurate enough to spot these variances. 
Additionally, an element's mass contribution to even a pure stoichiometric composition may vary due to the isotopic makeup of an element, which can change depending on its source. Since some environmental isotopes may be preferentially concentrated by astronomical, atmospheric, oceanic, crustal, and deep Earth processes, this variation is employed in radiometric dating. Except in the case of hydrogen & its isotopes, the effects are often negligible but can be measured with current technology. Even when considered "pure," many natural polymers (including DNA, proteins, and carbohydrates) have variable chemical compositions. Except in cases when their stoichiometry is consistent and their molecular weight is homogeneous (mono-disperse), polymers are typically not regarded as "pure chemical compounds." Due to isotope variances, they might still be breaking the law in this peculiar circumstance.
Next TopicLearning Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










