Limiting ReagentIn a balanced chemical equation, generally one of the reactants is present in a larger amount than the other. So, the amount of the product formed in such reactions depends on the reactant that reacts completely or is consumed completely in a chemical reaction. This reactant is called the limiting reactant. Whereas, the reactant, which is present in excess does not react completely or left unreacted. 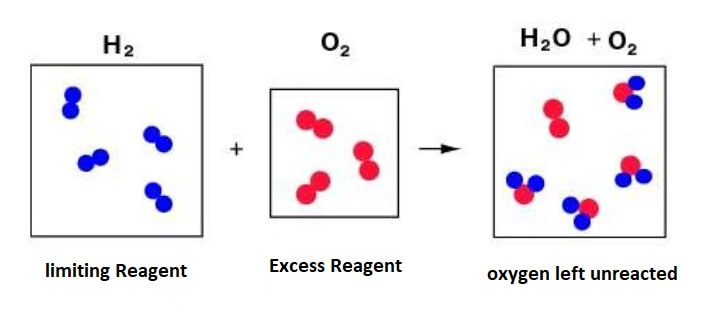
In this type of stoichiometry questions, the quantity such as mass or volume of two reactants is given and based on that we have to calculate the amount of product produced. Here, we have to choose the amount (mole, mass or vol.) of one of the reactants out of the given two reactants. So, in such questions, the solution starts from choosing the right reactant and using its details given in the question to find out the details of the product. The reason for choosing an appropriate reactant is that amount of the given reactants would not be in proportion in which they should be to produce the appropriate amount of product. Let us understand it with the following example; A + B → C + D A and B are reacting to form C and D In this reaction, we have to find out the amount of C or D or both produced in this reaction. We have given the masses of A and B, which are p1 and p2, respectively. Now, random quantity or masses of A and B cannot react to produce the specific amount of C and D. It means when a specific amount of A reacts with a specific amount of B, only then a specific or required amount of C and D will form. We can say that when p1 mass of A would be in some specific proportion or ratio with the p2 mass of B only then the specific amounts of C and D are formed. For example, p1 mass of A needs only 5% of p2 mass of B to form the C and D. The extra mass of p2 is not going to help anyway. So, when the reaction is complete, the remaining 95% mass of B, which is left behind unused or unreacted will not take part in the chemical reaction or in the formation of C and D. However, the entire mass of A will be consumed in this reaction. So, in this reaction, it is the A that takes the full responsibility to form C and D and it let itself consumed completely to form C and D. So, this reaction will continue until the mass of A is consumed completely. As soon as A is finished the reaction will stop and there will be no further formation of C and D even though B is not finished completely. So, a reactant is consumed completely in a reaction and decides how long the reaction will occur and how much products will be produced is called limiting reagent. So, in the above example, A is the limiting reagent. The reagent which is left unreacted is called excess reagent. So, in the above reaction B is the excess reagent. The word reagent is used for reactants who react to form products. Let us understand limited reagent with a simple chemical reaction between hydrogen and oxygen gas that leads to the formation of water, such as; 2H2 + O2 → 2H2O From the above balanced chemical reaction, it is clear that; 2 mole (4 gm) of hydrogen + (reacts with) 1 mole (32 gm) of oxygen → (to form) 2 moles or 36 gm of water Now, if someone takes 16 gm of oxygen gas and reacts it with 4 gm of hydrogen gas to produce 20 gm of water (2H2O) as 16 gm + 4 gm = 20 gm, what will happen? It will not produce 20 gm of oxygen. As for 4 gm of hydrogen, there should be 32 gm of oxygen to produce 36 gm of water, or 4 gm of hydrogen and 32 gm of oxygen react completely (none of them left unreacted) to form 36 gm of water. When the ratio between masses of hydrogen and oxygen is 4: 32 or 1: 8 they react completely. Now, we have only 16 gm of oxygen. It does not mean that reaction will not take place. The reaction will definitely take place but the amount of hydrogen that will react with the given amount of oxygen will be different. We can say that 4 gm of hydrogen is an excess amount for 16 gm of oxygen or 16 gm of oxygen is not sufficient or is less than required for the 4 gm of hydrogen. For 16 gm of oxygen, the amount of hydrogen required should be less than 4 gm or we can say that less amount of oxygen is present than it should be as per the requirement of hydrogen. So, either we have to increase the amount of oxygen or we have to use less amount of hydrogen. In this reaction, as oxygen is present in less amount and hydrogen is present in excess, so, oxygen will be consumed completely and the reaction will occur as long as 16 gm oxygen does not react completely. So, in this reaction, oxygen acts as or is the limiting reagent and hydrogen gas is excess reagent as it is more than required. Now, for 32 gm oxygen, 4 gm hydrogen gas was needed So, for 16 gm of oxygen, only 2 gm of hydrogen will participate in the reaction and form water accordingly, the reaming 2 gm of hydrogen will be left unreacted. So, 2 gm of hydrogen on reacting with 16 gm of oxygen will produce 18 gm of water (H2O). So, here, oxygen is deciding the quantity (mass or volume) of the product formed. Also, note that it is not necessary that reagent which is present in less amount will always be the limiting agent. As in the above example, hydrogen is less than oxygen but it is not the limiting agent. Let us solve some questions on Limiting Reagent Concepts:1) Question: In a reaction, A + B2 →AB2 Identify the limiting reagent in the following different conditions. i) 300 atoms of A + 200 molecules of B Solution: The given reaction is a balanced reaction as we have an equal number of atoms of A and B on both sides. A + B2 →AB2 Besides this, in this reaction, we can see 1 mole atoms of A (as A is in elemental form) + 1 mole molecules of B → 1 mole molecules of AB2 Or NA atoms of A are reacting or combines with NA molecules of B (NA = 1 mole particles or 6.023 x 1023 particles (Avogadro's number) So, for the given 300 atoms of A, we will need 300 molecules of B, but we have given 200 molecules of B, so we have less number of B molecules, which shows that in this reaction B is the limiting reagent and the reaction will continue until B is not consumed completely. ii) 2 mole of A + 3 mole of B In this condition, 2 moles of A are reacting with 3 moles of B. Now, we have known from the first question that for 1 mole of atoms of A, we will need 1 mole molecules of B So, for 2 moles of A, we will need just 2 moles of B, but we have given 3 moles of B, so it is clear that B is present in excess or A is less than required. Hence, A will be our limiting reagent. iii) 100 atoms of A + 100 molecules of B Solution. Now we have already found out the relation that 1mole atoms of A react with 1 mole molecules of B in this reaction. It shows, NA atoms of A reacts with NA molecules of B, here NA Avogadro's number or 6.022 x 1023 Now, 100 atoms of A will react with 100 molecules of B So, in this case, we have no limiting reagent, as both A and B will react or get consumed completing to form product AB2. They are present in exact proportion not more or nor less than required. A is exactly what B requires or B is exactly what A requires, which means for 100 atom A and the required 100 molecules of B are present, and vice versa. iv) 5 moles of A + 2.5 moles of B Solution: We know that 1 mole atoms of A react with 1 mole molecules of B in this reaction. So, 5 moles of A will need 5 moles of B, but we have given 2.5 moles of B so, B is less than required in this case and the reaction will continue as long as B is not consumed completely. Hence, B is a limiting reagent. v) 2.5 moles of A + 5 moles of B Solution: Now, as per the question, 2.5 moles of A are reacting with 5 moles of B We know that 1 mole atoms of A need 1 mole molecules of B in this reaction. So, for 2.5 moles of A, we will need 2.5 moles of B if we want nothing to be left unreacted. But, we have 5 moles of B, so, it will not be consumed completely, and its some part will be left unreacted. Whereas A will be consumed completely, so, we can say that A is less than required as compared to 5 moles of B and A also controls this reaction, so A is the limiting reagent. 2) Question: Dinitrogen (N2) reacts with dihydrogen (H2) to form ammonia (NH3) as shown below; N2 (g) + H2 (g) → 2NH3 (g) (i) Calculate the mass of ammonia produced when 2.00 x 103 g of dinitrogen reacts with 1.00 x 103 g of dihydrogen? Also, find out if there is any reactant that remains unreacted if yes find out its mass? Solution: Balance the chemical equation of this chemical reaction, as follows; N2 (g) + 3H2 (g) → 2NH3 (g) The balanced chemical equation shows that; The molar ratio of nitrogen and hydrogen, N2: H2 = 1: 3 So, one mole of N2 reacts with 3 moles of H2 (g) to produce 2 moles of NH3 (g) After converting moles into grams we get; 28 gm of N2 reacts with 6gm (3x2gm) of H2 Let us convert the above quantity into Kg as we have given quantities in 103 gm. 28 kg of N2 reacts with 6 kg of H2, which shows that 28 kg of N2 need 6 kg of H2 So, 1 kg of N2 will need (6 /28) kg of H2 As per the question, 2 kg dinitrogen will need (6/28) x 2 = 3/7 or 0.42 kg of dihydrogen (H2), but we have given 1 kg of dihydrogen (H2), which is more than required. So, N2 is the limiting reagent and H2 is the excess reagent in this reaction. As H2 is present in excess amount, it will not react completely or remain unreacted. Now let us calculate the mass of ammonia produced. The production of the product, which is ammonia in this reaction, depends on the amount of limiting reagent. Here, 2 kg of nitrogen gas reacts with 0.43 kg of hydrogen gas The mole ratio of limiting reagent nitrogen and ammonia, N2 and NH3 is 1: 2 It shows, 1 mole of nitrogen produces 2 moles of ammonia Or 28 gm of nitrogen produces 34 gm of ammonia Or 28 kg of nitrogen gas produces 34 kg of ammonia So, 2 kg of nitrogen gas will produce (34/28) x 2 = 17/7 = 2.4 kg or 2.4 x 103 gm of ammonia. Now, we were also asked that if there is any reactant that will be left unreacted. So, the answer is Yes as the hydrogen is more than required, it will not react completely. The mass of hydrogen gas that is left unreacted = total mass - reacted mass, 1 kg - 0.42 kg = 0.58 kg or 0.58 x 103 gm.
Next TopicSolution
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










