Malic AcidIt is an organic compound; a dicarboxylic acid found as an active ingredient in many sour and tart foods. This is because it is produced during fruit metabolism and thus is naturally present in almost all fruits and many vegetables. Its name malic is derived from the Latin word 'malum' which means apple. Further, it is produced in metabolic processes in the cells of plants, animals and humans. It provides energy to cells and the carbon skeleton needed for the formation of amino acids. Every day our body produces and breaks down a relatively large amount of malic acid. The sour taste of fruits is due to the malic acid. Owing to these properties, it is widely used as an artificial additive. Further, it has two stereoisomeric variants: L and D enantiomers. However, only the L-isomer is found in nature. In 1785, Malic acid was first described by Carl Wilhelm Sheele, a pharmaceutical chemist. Chemical Formula and Structure: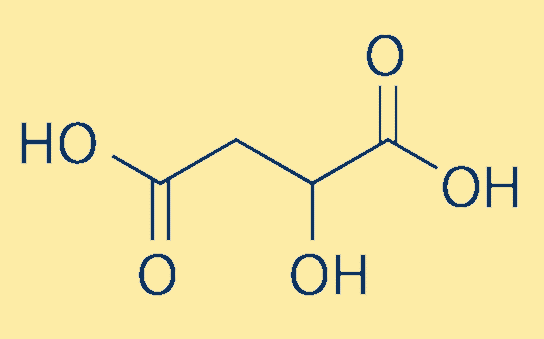
Malic acid formula is C4H6O5. Its salts are called malates. It is a 2-hydroxy dicarboxylic acid or 4-carbon dicarboxylic acid. Its IUPAC name is 2-Hydroxybutanediolic acid. It is the conjugate acid of malate (2- ) and malate. Further, it has an asymmetric carbon. Properties of Malic Acid
Preparation of Malic Acid:Although malic acid is produced in plants naturally, there is no cost-effective way of extracting malic acid from plants. This is the reason that it is manufactured. Malic acid is usually produced on a large scale in industries. It is produced from the maleic anhydride through its double hydration. In 2000, around 5000 tons of malic acid was produced in America annually. The main methods of production are as follows:
Among the above three methods, the fermentation method is the most cost-effective and environment-friendly way of manufacturing malic acid. Dosage and PreparationThere are no standard doses of malic acid that are recommended by doctors. Different doses are used in studies to find out the treatment of different conditions using malic acid. So, the appropriate dose for a person may depend on his or her age, gender, medical history, etc. So, one may speak to his or her doctor to find out the correct dosages of malic acid. Here are some doses that are studied in research for adults: For dry mouth: Mouth sprays that contain 1% malic acid are used nearly 8 times a day for 2 weeks. Lozenges that contain 28 mg malic acid are used 4 times a day for 6 months. Drug InteractionsThere are certain combinations that you should be aware of before taking malic acid. Here are they: Malic acid tends to lower blood pressure so it should not be taken along with medications for high blood pressure as blood pressure may go too low. PrecautionsThere is not enough evidence available to know if malic acid is safe to take during pregnancy or breastfeeding. So, you should stay on the safe side and don't take it in greater amounts. It tends to lower blood pressure so those who are prone to low blood pressure should avoid it as it may increase the chances of blood pressure becoming too low. Uses of Malic AcidMalic acid offers lots of uses in different fields. Some of its major uses are as follows: Skin Health: It is widely used in skincare products owing to its ability to help prevent and cure acne, wrinkles, warts, hyperpigmentation, calluses, etc. Further, it aids the shedding of the outer layer of skin and thus provides anti-ageing benefits. Oral Health: It helps prevent xerostomia (dry mouth) owing to its ability to stimulate the production of saliva in the mouth. The increased saliva also helps prevent bacterial overgrowth inside the mouth. Improves Absorption of Iron: Iron is an important nutrient for good health, especially for pregnant women and people who are suffering from anemia. It has been found in a study that malic acid and vegetable-rich vitamin C help improve the absorption of iron in our bodies. Boosts Physical Performance: When taken as a supplement, it helps boost physical performance such as performance in sports or athletic performance. Further, it also keeps muscles from exhausting during exercise. It is also taken in combination with creatine to gain lean muscle mass. Kidney Stones: It helps in the formation of citrate, which keeps calcium from binding with other substances in urine that tend to form kidney stones. Fibromyalgia: According to a study, when malic acid is taken with magnesium it provides relief from pain and tenderness caused by fibromyalgia. Food: It is used as a food additive to impart a sour taste to food items. Further, it is used to produce low-calorie beverages including iced tea, sports drinks, etc., owing to its ability to dissolve rapidly and enhance flavour. For example, malic acid can provide more sourness than citric acid, so less acidulant is needed. It is also cheaper than citric acid. Cosmetics: It is used to control the acidity of cosmetic products. For example, many cosmetic products like facial cream, self-tanning cream, etc., contain malic acid as a pH regulator. Energy: Malic acid takes part in the Krebs cycle, a process that produces energy in the body required to perform various biological functions that keep us alive. Ciders and Wines: It is used to provide a consistent sharp taste to alcoholic apple ciders. Further, it aids fermentation, which helps improves the flavour of wines. Calcium Supplements and Beverages: It is used in liquid calcium supplements to give them tart and fruity flavour along with controlling their pH. Further, in calcium-rich drinks, malic acid when used as an alternative for citric acid prevents turbidity that arises due to precipitated calcium citrate. Biomedical applications: Macromolecular substances obtained from I-malic acid are used for biomedical applications. Unfamiliar facts about Malic AcidThe intake of malic acid in food is considered safe and healthy and does not cause any harmful side effects. However, when taken as a supplement or as a synthetic form it may cause side effects. For example, malic acid candies when consumed may irritate the mouth, throat and stomach. Further, if you eat something too much that contains malic acid it may irritate your mouth and may cause diarrhea, nausea, allergic reactions, and acidity in some cases. Additionally, it may cause irritation to the skin and eyes when it is applied to the skin. Difference between Malic Acid and Maleic AcidAlthough both are dicarboxylic acids, they are different from each other. Some of the major differences between them are as follows:
Malic Acid SafetyMalic acid has been given a GRAS designation by the United States Food and Drug Administration (FDA), which shows that it poses little to no health risk when consumed at normal levels. Further, the Environmental Protection Agency (EPA) has awarded it 'green cycle' which means that United States EPA does not think that it could be harmful. Further, some studies suggest that the intake of high doses of malic acid for up to six months does not cause any adverse effects. However, malic acid should not come in contact with your eyes and skin. Beyond that, it is safe to consume.
Next TopicTypes of Alcohol
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










