Melting Point DefinitionThe temperature at which a substance transitions from a solid to a liquid state is known as its melting point (or, less frequently, liquefaction point). Solid and liquid phases are equally present at the melting point. Pressure affects a substance's melting point, which is typically reported at a normal atmospheric pressure, such as 1 atmosphere, 100 kPa. The term "freezing point" or "crystallization point" refers to the temperature at which a liquid returns to a solid. Due to a substance's capacity for supercooling, the freezing point might frequently appear to be lower than it actually is. In actuality, the actual process used to identify a substance's characteristic freezing point is nearly invariably "the principle of detecting the disappearance rather than the production of ice", that is, the melting point. 
ExamplesThe melting & freezing points of the majority of substances are roughly comparable. For instance, mercury has a melting & freezing point of 234.32 kelvins (38.83 °C; 37.89 °F). Yet, the temperatures at which some compounds change from solid to liquid vary. For instance, agar solidifies at 31 °C (88 °F) and melts at 85 °C (185 °F); this direction dependency is known as 'hysteresis'. The ice point, commonly known as 0 °C (32 °F; 273 K), is the temperature at which ice melts at 1 atmosphere of pressure. When nucleating chemicals are present, water's freezing and melting points aren't necessarily the same. Water can be supercooled to a temperature of 48.3 °C (or 54.9 °F; 224.8 K) prior to actually freezing in the case of a lack of nucleators. Tungsten has the greatest melting point of any metal, which is 3,414 °C (6,177 °F; 3,687 K), making it a perfect material for use as incandescent lamp filaments. The frequently quoted carbon doesn't melt under atmospheric pressure; instead, it sublimes at a temperature of roughly 3,700 °C (6,700 °F; 4,000 K). A liquid phase only develops above pressures of 10 MPa (99 atm) and an approximated 4,030-4,430 °C (7,290-8,010 °F; 4,300-4,700 K). The only substance proven to have a melting point exceeding 4,273 K (4,000 °C; 7,232 °F) at atmospheric temperature is the refractory compound hafnium carbonitride (HfCN), which has the highest melting point of any substance known to exist as of yet. The melting point of HfN0.38C0.51 was anticipated by quantum mechanical computer models to be around 4,400 K. Although an accurate measurement of its melting point has not yet been verified, this assumption was later verified by experiment. In contrast, even at temperatures very close to absolute zero, helium somehow doesn't freeze at all under normal pressure; more than 20 times the atmospheric pressure is required. Measurements of melting pointsThere are numerous laboratory methods available for figuring out melting points. A metal strip with a gradient in temperature from ambient temperature to 300 °C is referred to as a Kofler bench. Any material can be applied to a piece of the strip to display its thermal behavior at that temperature. Melting point and enthalpy of fusion information are provided by differential scanning calorimetry. A basic oil bath with a clear window (the simplest design is a Thiele tube) and even a straightforward magnifier make up a melting point analysis tool for crystalline substances. A narrow glass tube containing a number of granules of a solid is submerged in an oil bath. The oil bath is heated and agitated, and using a magnifier and an external light source, it is possible to see the melting of the small crystals at a specific temperature. An oil bath could be replaced with a metal block. Automatic optical detection is a feature of some contemporary instruments. Using an operational procedure, the test can also be conducted continually. For instance, diesel fuel's freeze point is tested "live" at oil refineries, which means that a sample is automatically obtained from the process & recorded. As the sample does not need to be manually extracted and transported to a distant laboratory, more frequent measurements are now possible. Refractory materials techniquesThe exceptionally high melting point of refractory materials, such as platinum, tungsten, tantalum, certain carbides, and nitrides, etc., can be identified by the material being heated in a black body furnace and monitoring the black-body temperature using an optical pyrometer. This may call for exaggeration by several hundred degrees for the materials that melt at the greatest temperatures. It is well known that an incandescent body's spectral brightness depends on its temperature. An optical pyrometer compares the brilliance of a target object to the source's radiance, which has already been calibrated as a factor of temperature. This eliminates the need to estimate the radiation intensity's absolute magnitude. However, the calibration of the pyrometer must be established using known temperatures. A technique known as extrapolation must be used for temperatures that are higher than the source's calibration range. Using Planck's equation of radiation, this extrapolation is performed. The extrapolation errors increase with temperature because the constants in this equation cannot be determined with sufficient precision. But regular methods for performing this extrapolation have been devised. 
Consider the scenario when the source is gold (mp = 1,063 °C). In this method, the current flowing through the pyrometer's filament is changed until the light output of the filament is equal to the light output of a black body at the temperature of gold melting. It is possible to define this in terms of the current flowing through the pyrometer bulb, and it establishes the primary calibration temperature. The pyrometer detects a different black body with a greater temperature using the same current settings. The pyrometer is placed in front of this black body, and between them is an absorbing material with the known transmission. The black body's temperature is then changed until the pyrometer filament's intensity and intensity are equal. The black-actual body's higher temperature is then calculated using Planck's Law. After removing the absorbing material, the filament's current is adjusted such that it has the same intensity as the black body. As a result, the pyrometer has a second calibration point. To calibrate the instrument at a higher temperature, this step is repeated. Now that temperatures and the pyrometer filament currents that correlate to them are known, a temperature vs. current curve may be created. Then, the curve can be extended to extremely high temperatures. This method requires either black body conditions or knowledge of the material's emissivity in order to calculate the melting points of refractory materials. Experimental challenges could arise from keeping the high melting material in the liquid. In order to determine the melting points of several refractory metals, solid metal specimens that were significantly longer than they were wide were used to observe the radiation coming from a black body cavity. A hole is drilled near the centre of a rod of the material, perpendicular to the long axis, to create the hollow. A very strong current is then used to heat these rods, and an optical pyrometer detects the radiation coming from the hole. When the liquid phase emerges and displaces the black body conditions, the hole darkens, signalling the melting point. Nowadays, fast pyrometers and spectro-pyrometers are used in conjunction with container less infrared heating techniques to precisely control the amount of time the sample is exposed to high temperatures. These short-duration tests address a number of problems with more conventional melting point measurements carried out at very high temperatures, including sample vaporization and container reactivity. ThermodynamicsThe melting point of water is pressure dependent. It takes heat to bring a solid's temperature up to the melting point, which causes it to melt. The melting must be accompanied by additional heat, known as the heat of fusion, which is an example of latent heat. From a thermodynamics perspective, the material's Gibbs free energy (G) changes nothing at the melting point, but its enthalpy (H) and entropy (S) increase (H, S > 0). Melting occurs when the liquid's Gibbs free energy is smaller than the solid's for a given material. This occurs at a particular temperature and a variety of pressures. It can be demonstrated that: 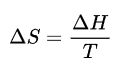
△H=T, and △S=frac are equal. Here, T, S, and H stand for the melting point temperature, entropy change during melting, and enthalpy change during melting, respectively. Although the melting point is sensitive to extremely large changes in pressure, it is often orders of magnitude less sensitive than the boiling point since the transition from solid to liquid only involves a tiny change in volume. The melting point will rise as the pressure increases if, as is typically observed, a substance is denser in the solid than in the liquid state. Otherwise, the opposite behavior takes place. Interestingly, this is also the case for Si, Ge, Ga, and Bi, as graphically shown to the right. The melting point is seen to alter significantly with exceptionally massive pressure variations. For instance, silicon has a melting point of 1415 °C at atmospheric pressure (0.1 MPa), but this temperature drops to 1000 °C at pressures greater than 10 GPA. 
Melting points are frequently used to describe and evaluate the purity of organic and inorganic substances. Compared to an impure substance's melting point or, more broadly, the melting point of mixtures, a pure substance's melting point is always higher and has a narrower range. The melting point range, also known as the "pasty range," will be wider, and the melting point will be lower the more additional components there are in the mixture. The solidus is the temperature at which a mixture starts to melt, while the liquids are the temperature at which melting is complete. Certain combinations called eutectics exhibit a single phase-like behavior. They quickly dissolve into a liquid with the same composition when the temperature remains steady. As an alternative, a liquid with a eutectic composition will cool and crystallize as uniformly scattered, small (fine-grained), mixed crystals. Glasses do not have a melting point, in contrast to crystalline solids, and when heated, they smoothly transform from glass to a viscous liquid. They gradually soften with additional heating, which is characterized by specific softening points.
Next TopicMotion- Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










