Metalloid Definition
Metalloids are a class of chemical elements with characteristics of both metals and non-metals, thus called "semimetals." They are situated between the non-metals on the right and the metals on the left in the "staircase" section of the periodic table. The elements boron (B), germanium (Ge), silicon (Si), arsenic (As), antimony (Sb), & tellurium are frequently referred to as metalloids (Te). The metalloids are located below the line in certain periodic tables that different metals and non-metals.
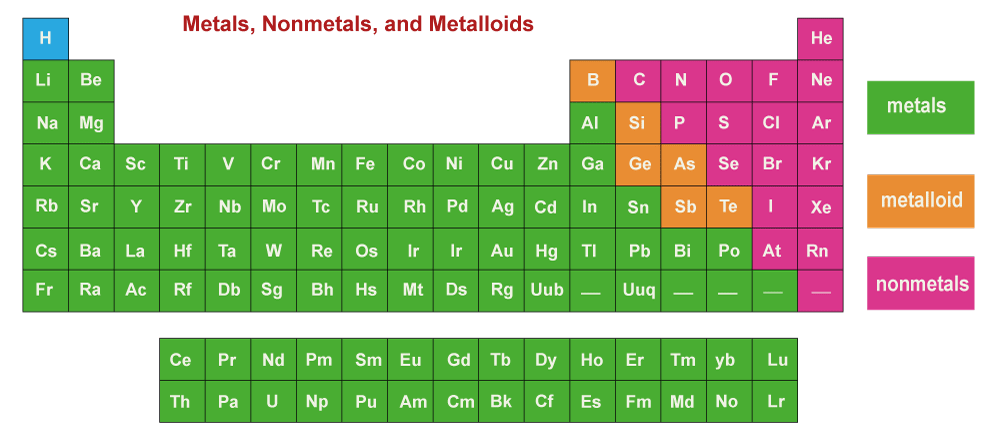
Properties of Metalloids
Metalloids are a class of elements that display characteristics of both metals as well as non-metals. They are sometimes referred to as semimetals. These are some of the metalloids' distinctive properties:
- Electrical conductivity- While metalloids have some electrical conductivity, they are typically less than metals. To create semiconductors, which are essential in electronics, it is crucial to have this feature.
- Thermal conductivity- Metalloids typically have a thermal conductivity that is higher than non-metals but lower than metals. This characteristic is crucial for creating insulators regulating heat flow in various applications.
- Ductility- Metalloids are often more ductile than non-metals but less ductile than metals. This property is crucial in creating cables and other flexible components.
- Brittle- Metalloids are often brittle, which means they are susceptible to breaking or shattering when stressed. In the manufacture of ceramics as well as other materials that demand great rigidity and strength, this property is crucial.
- Oxidation states- Metalloids have a wide range of oxidation states, allowing them to produce a large range of chemical compounds. This characteristic is crucial for the manufacture of semiconductors since they require precise chemical reaction control.
- Reactivity- Metalloids display a wide range of reactivity depending on their unique characteristics and chemical surroundings. Many chemical processes, such as catalysis and chemical synthesis, depend on this property.
- Allotropy- Metalloids can live in several crystal forms with various physical and chemical characteristics. When creating materials with certain qualities and properties, this attribute is crucial.
- Solubility- Metalloids have varied solubility in different solvents, allowing them to be used in various applications.
- Electronegativity- Metalloids have intermediate electronegativity values, which means they may form both covalent & ionic bonds with other elements.
- Optical properties- Certain metalloids have unique optical properties, such as the capacity to absorb & emit light at specific wavelengths. This property is significant in the manufacture of sensors and other light-based devices.
Applications of Metalloids
Semiconductors- Metalloids like germanium and silicon are frequently used to manufacture semiconductors and other electronic parts. They are essential to the operation of contemporary electronics because, under certain circumstances, they can conduct electricity.
- Glass manufacturing- Borosilicate glass, recognized for its strength, durability, and heat resistance, contains boron, a crucial component. Wide-ranging uses for this kind of glass include lighting, cookware, and scientific equipment.
- Agriculture- Boron is a micronutrient that plants absolutely must have. It is frequently added to fertilizer to increase crop quality and productivity.
- Construction- To boost cement and concrete's strength, durability, and corrosion resistance, metalloids like boron & silicon are added as additives.
- Construction- To boost cement and concrete's strength, durability, and corrosion resistance, metalloids like boron & silicon are added as additives.
- Energy production- To increase the performance and efficiency of solar panels and other electrical equipment, antimony is employed as a doping agent during manufacturing.
- Pyrotechnics- Bright colors and distinctive effects that make these displays so popular are produced by employing metalloids like antimony and bismuth to manufacture fireworks and other pyrotechnic devices.
- Flame-retardants-Boron and antimony are used in an array of products, such as textiles, polymers, and electronics.
- Water treatment- To eliminate impurities from drinking water and wastewater, arsenic is used to manufacture compounds that treat water.
- Medicine- Bismuth, a metalloid, has been proven to have antibacterial characteristics and is used in several treatments to treat digestive issues.
- Imaging- Germanium is used to make X-ray & gamma-ray detectors, which are used in a variety of applications, including security screening and medical imaging.
Formation of Metalloids
The metalloid formation is determined by the conditions in which they are formed.
In general, covalent and ionic bonds between the metal & non-metal atoms combine to generate metalloids. As a result, a hybrid structure with characteristics of both metals and non-metals is formed.
For example, silicon, a metalloid, is created when silicon and oxygen combine through covalent bonding to form silicon dioxide (SiO2). The resultant compound possesses the characteristics of a metalloid because of its intermediate crystalline structure between that of a metal and a non-metal.
Another example is boron, a metalloid created by the covalent bonding of boron atoms. Its distinct atomic structure makes it difficult to categorize it as a metal or a non-metal.
How are Metalloids Identified?
Metalloids are distinguished by their position on the periodic table, which is on the threshold between metals and non-metals. They display amphoteric behavior, have a partly filled valence shell, and intermediate properties between metals & non-metals. They may also produce both acidic and basic oxides. Metalloids may create both ionic and covalent connections with other elements due to their intermediate electronegativity. They may interact with bases as well as acids to generate compounds with non-metals and alloys with metals. To ide
Drawbacks of Metalloids
Listed below are some drawbacks of metalloids:
Toxicity- Some forms of metalloids, such as arsenic and antimony, may harm people and the environment. These substances can seriously harm your health and cause cancer, brain damage, and reproductive issues.
Brittleness- Metalloids are often brittle, which means that stress can cause them to break. Because of this, they are inappropriate for use in several applications, such as structural materials.
Limitations of semiconductors- Metalloids are semiconductors, and their conductivity is limited compared to actual metals. Their utility in electronic applications may be constrained as a result.
Reactivity- Under some circumstances, metalloids can be quite reactive, which can make handling and using them challenging.
Incompatibility- Metalloids may not work well when combined with specific metals or other materials, which may restrict their utility in some situations.
Facts of Metalloids:
Listed below are some metalloids' facts:
- Metalloids are also known as semimetals or semiconductors because they have qualities of both metals and non-metals.
- Baron Jöns Jakob Berzelius, a Swedish scientist, created the term "metalloid" for the first time in 1828.
- Metalloids have different electronegativities, meaning they may form ionic and covalent bonds.
- Metalloids play a crucial role in manufacturing microchips, solar panels, or other electrical equipment, among other technical applications.
- Some kinds of metalloids can be hazardous to humans and the environment. For example, arsenic is a hazardous metalloid that, in large amounts, can cause major health issues.
- The only metalloid found in vitamins is boron.
- Solar cells are made with the help of tellurium.
- Nuclear bombs have used polonium, a rare and extremely radioactive metalloid.
- Metalloids and metals can combine to create alloys like silicon bronze.
- Amphoteric behavior, a special characteristic of metalloids, refers to their capacity to function as both bases and acids.
- Although arsenic is used to make insecticides and cure wood, large amounts of the substance may be hazardous.
- Rewritable DVDs are created using Tellurium.
- Minerals like germanite and borax include metalloid compounds that can be found in nature.
- Metalloids are essential in materials science and nanotechnology because of their unique features and future uses.
The Conclusion
Metalloids are significant substances that are essential to many contemporary technologies. They have distinct qualities that make them perfect for particular purposes, and their capacity to function as electrical semiconductors has made them very useful in the electronics industry. Metalloids are expected to play an increasingly bigger role in determining the future of technology & industry as our understanding of materials as well as nanotechnology advances.
|

 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










