Nitric AcidNitric acid is a strong acid. Its chemical formula is HNO3 and is also called the spirit of niter and aqua fortis. It is colorless when exists in pure form but turns yellow when it gets older. The yellow color is the result of the decomposition of nitric acid into oxides of nitrogen and water. Nitric Acid Structure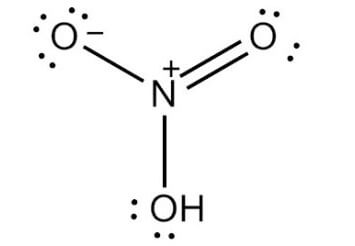
The molecule of nitric acid is made of 3 oxygen atoms, 1 nitrogen atom, and 1 hydrogen atom. In its molecule, one of the oxygen atoms is bonded to the central nitrogen atom through double bonds. While the other oxygen atom is bonded to the central nitrogen atom and hydrogen atom through a single bond. The third and the last oxygen molecule has a -1 charge and is bonded to central nitrogen through a single bond. As the central nitrogen atom takes part in four covalent bonds with three oxygen atoms, it has a +1 charge. So, the nitric acid has 0 net charge as the positive charge on the nitrogen atom is cancelled or neutralized by the negative charge on the third oxygen atom. The molecule of Nitric acid has a planar shape in which the nitrogen atom is bonded to three oxygen atoms; out of which, one oxygen atom holds the proton. The remaining two N-O bonds are equivalent and display the resonance with double bond character. Physical properties of Nitric Acid (HNO3)
Chemical Properties of Nitric Acid1. It is a strong monoprotic or monobasic acid. It ionizes in an aqueous solution as shown below: HNO3 + H2O → H3O+ + NO-3 It also reacts with metallic oxides, hydroxides, carbonates and bicarbonates to produce nitrates, for example: CaO + 2HNO3 → Ca (NO3)2 + H2O NaHCO3 + HNO3 → NaNO3 + H2O + CO2 Na2CO3 + 2HNO3 → 2NaNO3 + H2O + CO2 2. It changes blue litmus to red. It easily forms solid hydrates like monohydrate HNO3.H2O and the trihydrate HNO3.3H2O 3. In the presence of light or when heated, it decomposes easily and forms brown nitrogen dioxide. So, fresh nitric acid, which is white, turns brownish over time. The reaction is given below: 4HNO3 + heat → 2H2O + 4NO2 + O2 4. It is a strong oxidizing agent as it produces nascent oxygen both in the concentrated as well as in the dilute form. For example: 2HNO3 (conc.) → 2NO2 + H2O + (O) 2HNO3 (dil.) → 2NO + H2O + 3 (O) Thus, it also quickly reacts with various non-metallic compounds and oxidizes them. For example: C+4HNO3 → 4NO2 + 2H2O + CO2 3H2S + 2HNO3 → 3S + 4H2O + 2NO 5. Hydrogen gas is released when nitric acid reacts with metals located above hydrogen in the metal activity series. For example: Mg + 2HNO3 → Mg (NO3)2 + H2 Mn + 2HNO3 → Mn (NO3)2 + H2 Uses of the Nitric Acid
Preparation of Nitric AcidLaboratory preparation of Nitric Acid:In this method, nitric acid is prepared in the lab by heating a nitrate salt such as sodium nitrate, potassium nitrate, etc., with concentrated sulphuric acid, in a glass reservoir. The chemical reaction is shown below: NaNO3 + H2SO4 + Heat → NaHSO4 + HNO3 The nitric acid vapors produced in the reaction are condensed to form brown liquid using a reservoir that is cooled under cold water. Commercial production of Nitric AcidAs nitric acid is very important commercially, it is also produced in large quantities through commercial production. Generally, the cost-effective methods that ensure higher yield are preferred. Some of which are described below: 1) Birkland and Eyde Process: This process works as described below: 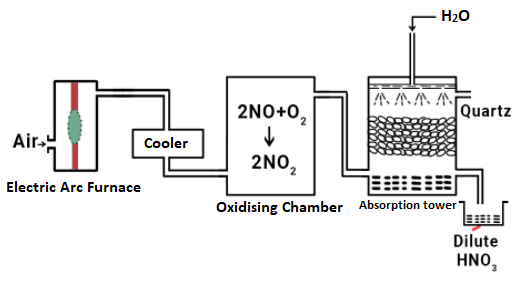
i) The air is passed through the electric arc to make nitrogen react with oxygen. The reaction is shown below: N2 + O2 → 2NO + energy The reaction that takes place between N2 and O2 is endothermic and reversible. Further, high temperature is preferred for the formation of NO as per the Le Chatelier Principle. So, a temperature of around 3000 degrees Celsius is maintained by the electric arc. ii) NO that is formed is quickly cooled to 1000 degrees Celsius to prevent its decomposition. iii) Now, NO, which is produced combines with oxygen to form NO2 as shown below: 2NO + O2 → 2NO2 iv) The NO2 vapors are made to pass through water to produce HNO3. 2NO2 + H2O → HNO3 + HNO2 3HNO2 → HNO3 + 2NO + H2O 2) Ostwald's Process The set-up of Ostwald's process comprises a catalyst chamber or converter, oxidation tower and absorption tower. Converter: It is made of aluminum, it contains a platinum-rhodium gauze cylinder that is closed at the bottom using a silica lid. The gauze is heated to 800 degrees Celsius. Then the mixture of ammonia and air mixture enters through the gauze from the top and exit is present at the bottom to release the product. Cooler: The nitric oxide coming out of the converter is cooled down here. Oxidation Chamber: In this chamber, oxidation of nitric oxide (NO) to nitrogen dioxide (NO2) takes place. Absorption Tower: NO2 enters the absorption tower from the lower part and water is made to enter from the top. In this tower, nitrogen dioxide is absorbed into the water. 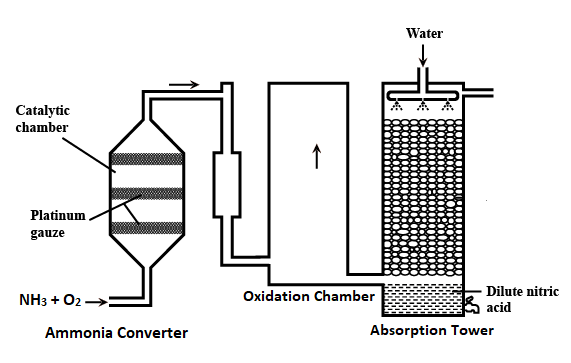
The reactions involved in Ostwald's process are as follows: i) The mixture of ammonia and air in the ratio of 1:10 is passed into the catalyst chamber over a Pt gauze catalyst at 750 to 900 degrees Celsius. It leads to the oxidation of ammonia to NO, whose yield is more than 90%. The reaction is shown below: 4NH3 + 5O2 → 4NO + 6H2O + Energy Further, the oxidation of ammonia in this reaction is exothermic as it occurs with the release of energy. ii) The NO produced is cooled and is mixed with air in the oxidizing chamber. It causes oxidation of NO to NO2, which is then cooled to 50 degrees Celsius. 2NO + O2 → 2NO2 iii) Now, NO2 is absorbed into the water in the absorption tower in the presence of air. 4NO2 + H2O + O2 → 4HNO3 The Nitric acid produced in the above step is concentrated to about 68% by distillation. Further, it can be made more concentrated up to 98% by distillation with concentrated H2SO4.
Next TopicHydrochloric Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










