Non-Metal DefinitionThe periodic table consists of 118 elements, divided into metals, nonmetals, and metalloids. Nonmetals are a group of elements that share several chemical properties, including not conducting electricity or heat, not being malleable, and not having a metallic lustre. They are located on the right side of the periodic table, excluding hydrogen, and are divided into four categories: Noble Gases, Halogens, Hydrogen, and Other Nonmetals. 
Definition of NonmetalsNonmetals are elements that do not possess the properties of metals. They are located in the upper right corner of the periodic table, excluding hydrogen, and consist of gases, liquids, and solids. Nonmetals are often characterized by their ability to form covalent bonds, low melting and boiling points, and lack of metallic lustre. They are usually poor conductors of heat and electricity, although exceptions such as carbon exist. Nonmetals are crucial components of living organisms, compounds, and materials such as plastics and semiconductors. Properties of NonmetalsNonmetals have several defining properties that distinguish them from metals. These include: 1. Electrical ConductivityNonmetals generally lack metallic properties, such as ductility, malleability, and the ability to conduct electricity. This is primarily due to their electron configuration, which is characterized by the presence of valence electrons in their outermost shell. Unlike metals, which have a few valence electrons that are free to move within the lattice structure, nonmetals have a full or nearly full valence shell and no free electrons. Since the ability to conduct electricity depends on the presence of free electrons that can move through a material, nonmetals, with their full or nearly full valence shell, are generally poor conductors of electricity. This lack of free electrons also makes them poor conductors of heat, as the movement of heat requires the transfer of energy through the movement of free electrons. While nonmetals are generally poor conductors of electricity, there are some exceptions. For example, in graphite, carbon can conduct electricity due to its unique structure, allowing the free electrons to its layers. However, overall, the lack of free electrons in nonmetals makes them poor conductors of electricity and limits their use in electrical applications. 2. Thermal ConductivityThe ability of a material to conduct heat depends on the movement of energy through its lattice structure. This movement is primarily facilitated by free electrons that can transfer energy through collisions with other particles. However, nonmetals generally lack free electrons in their outermost shell and are thus poor conductors of heat. Nonmetals have a different electron configuration than metals, which results in a full or nearly full valence shell. In contrast, metals have only valence electrons that can move through the lattice structure, facilitating energy flow. The lack of free electrons in nonmetals means the energy cannot be easily transferred through the lattice structure, resulting in poor heat conduction. Additionally, nonmetals tend to have weak interatomic forces, making them less dense and compact than metals. These weak forces further inhibit energy transfer through the lattice structure and reduce the material's ability to conduct heat. There are, however, some exceptions to this general trend. For example, a diamond, a form of carbon, has a unique lattice structure that allows it to conduct heat exceptionally well despite being a nonmetal. Similarly, some nonmetallic compounds, such as ceramics, can have high thermal conductivity due to their crystal structure. Overall, the lack of free electrons and weak interatomic forces make nonmetals poor conductors of heat. While some exceptions exist, nonmetals, such as electronics and thermal management, are generally not used in applications where heat conduction is critical. 3. Malleability And DuctilityMalleability and ductility are properties of materials that describe their ability to be deformed or reshaped without breaking. Metals are generally malleable and ductile, so they can be hammered into thin sheets or drawn into wires without breaking. On the other hand, nonmetals lack these properties, making them unsuitable for these types of applications. One reason is the difference in atomic structure between metals and nonmetals. Metals have a unique crystal structure that allows for the movement of electrons through the lattice structure, which results in their malleability and ductility. The movement of electrons makes it possible for the metal to be reshaped without breaking, as the electrons can flow through the lattice structure and maintain the material's structural integrity. In contrast, nonmetals generally have a different crystal structure that lacks the free-flowing electrons required for malleability and ductility. Nonmetallic materials typically have strong covalent bonds that hold the atoms together, which makes them more rigid and brittle than metals. When a nonmetal is subjected to force or pressure, the covalent bonds are more likely to break, resulting in the material's fracture rather than reshaping. The lack of free-flowing electrons and stronger covalent bonds make nonmetals unsuitable for malleable or ductile applications. While some exceptions exist, such as graphite, a form of carbon that can be used as a lubricant due to its malleability, nonmetals are generally not used in applications requiring these properties. 4. LustreThe characteristic metallic lustre of metals is due to the way that metals reflect light. Metals have a unique arrangement of electrons that allows them to absorb and re-emit light to create a shiny, reflective surface. This property is not present in nonmetals, which results in their dull or powdery appearance. Nonmetals generally have a different electron configuration than metals, making it difficult to absorb and re-emit light in a way that creates a metallic lustre. Nonmetals tend to have full or nearly full valence shells, meaning their electrons are tightly bound and not free to move through the lattice structure as they are in metals. This results in a different light absorption and reflection mechanism that does not produce a metallic lustre. In addition to their electron configuration, nonmetals tend to have weaker interatomic forces than metals, which results in their softer and more powdery appearance. The weaker interatomic forces make it easier for the material to break apart or crumble, which results in a less shiny and reflective surface. Overall, the lack of free-flowing electrons, weaker interatomic forces, and different mechanisms of light absorption and reflection make nonmetals unable to exhibit the characteristic metallic lustre that metals exhibit. While some nonmetals may have other unique properties that make them desirable for certain applications, their dull or powdery appearance makes them less suitable for applications that require a shiny or reflective surface. 5. Melting and Boiling PointsNonmetals and Metals are two main types of elements that are differentiated based on their properties, including physical and chemical characteristics. One of the most significant differences between nonmetals and metals is their melting and boiling points, with nonmetals generally having lower values than metals. The reason behind this difference is how the atoms of nonmetals and metals are bonded. In metals, the atoms are held together by strong metallic bonds, which result from the sharing of electrons among the atoms. These bonds form a lattice structure that allows for the efficient transfer of electrons, resulting in high electrical conductivity and flexibility. On the other hand, nonmetals tend to form covalent bonds with other nonmetallic elements, forming molecules. Covalent bonds involve the sharing of electrons between two atoms, which results in a stable bond. However, these bonds are weaker than metallic bonds, so less energy is required to break them apart, resulting in lower melting and boiling points. Another factor contributing to nonmetals' lower melting and boiling points is their relatively smaller atomic sizes. Nonmetals tend to have smaller atoms, with fewer electrons in their outermost electron shell. This means they have weaker electrostatic forces between their atoms, resulting in lower boiling and melting points. Furthermore, nonmetals tend to have a wider range of molecular structures than metals, resulting in various physical properties. For example, some nonmetals, such as sulfur and phosphorus, form molecules with complex structures, which can result in different types of intermolecular forces that affect their melting and boiling points. 6. ElectronegativityElectronegativity measures an atom's ability to attract electrons toward itself when it forms a chemical bond with another atom. The electronegativity values of elements can vary significantly and play a crucial role in determining the chemical properties of elements. Nonmetals generally tend to have higher electronegativity values than metals, meaning they have a stronger attraction for electrons. The electronegativity of an atom is affected by several factors, including its atomic size, the number of electrons in its outermost shell, and its nuclear charge. Nonmetals tend to have smaller atomic sizes, meaning their outermost electrons are closer to the positively charged nucleus. This increases the electrostatic attraction between the electrons and the nucleus, leading to higher electronegativity values. In contrast, metals tend to have larger atomic sizes, and their outermost electrons are further away from the nucleus. This results in weaker electrostatic forces between the electrons and the nucleus, leading to lower electronegativity values. Another factor contributing to nonmetals' higher electronegativity values is the number of electrons in their outermost shell. Nonmetals tend to have fewer electrons in their outermost shell, and they tend to have a high affinity for electrons to fill their valence shell. This results in a stronger attraction for electrons and a higher electronegativity value. On the other hand, metals tend to have more electrons in their outermost shell and a relatively low affinity for electrons. This results in a weaker attraction for electrons and a lower electronegativity value. The electronegativity values of elements play a critical role in determining the nature of chemical bonding between them. Nonmetals tend to form covalent bonds with other nonmetals because they have similar electronegativity values, resulting in a shared electron pair. In contrast, metals tend to form ionic bonds with nonmetals because of the significant difference in electronegativity values, resulting in the transfer of electrons from the metal to the nonmetal. 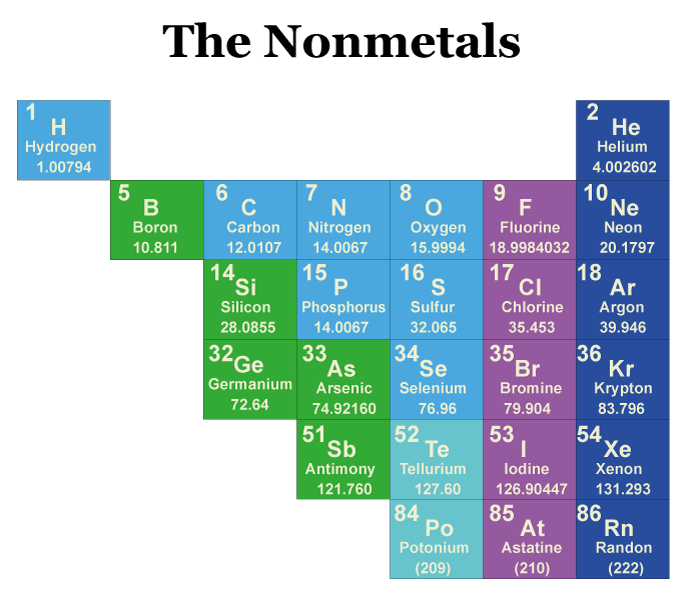
Types of NonmetalsNonmetals are divided into four categories: Noble Gases, Halogens, Hydrogen, and Other Nonmetals. 1. Noble GasesNoble gases, or inert gases, are six unreactive elements. They are located in Group 18 of the periodic table and include helium, neon, argon, krypton, xenon, and radon. Noble gases have a full outer shell of electrons, which makes them stable and unreactive. They are often used in lighting and welding applications. 2. HalogensHalogens are five highly reactive elements in Group 17 of the periodic table. They include fluorine, chlorine, bromine, iodine, and astatine. The halogens are very reactive because they have seven valence electrons and only need one more electron to complete their outer shell. They are used in various applications, including disinfectants, bleaches, and pesticides. 3. HydrogenHydrogen is a unique nonmetal in that it has properties of both metals and nonmetals. It is located at the top of Group 1 of the periodic table. It is often considered a nonmetal because it has a low boiling point and is a poor conductor of electricity. However, hydrogen also has properties of metals, such as its ability to form positive ions. Hydrogen is the most abundant element in the universe and is used in various applications, including fuel cells and rocket propellants. 4. Other NonmetalsElements like carbon, nitrogen, oxygen, phosphorus, sulfur, and selenium are among the other nonmetals. These substances are found in different periodic table groups and have special qualities crucial in various applications.
Industrial Use of NonmetalsNonmetals are widely used in various industries due to their unique chemical and physical properties. Here are some examples of how nonmetals are used in industry:
Their unique properties make them valuable in various industrial applications, and they will continue to play a critical role in developing new technologies and products. ConclusionNonmetals are various elements with distinctive characteristics and effective uses in chemistry, biology, and industry. For scientists and engineers working in these domains, understanding the features and properties of nonmetals is vital. Nonmetals are similar in many ways but have distinctive qualities that make them significant in certain applications. Scientists can create new technologies and materials to enhance our quality of life and deepen our understanding of the universe by researching nonmetals and their characteristics.
Next TopicProducer Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










