Normality Definition ChemistryThe term "normality" in chemistry describes the number of reactive substances or the concentration of a solution expressed in terms of its chemical equivalents. In acid-base and redox reactions, normality is a typical approach to gauge a solution's capability for reaction. Study of The History of NormalityChemists first began to investigate the quantitative features of chemical processes in the late 19th century, when the notion of normalcy in chemistry first emerged. Before this, qualitative descriptions of chemical reactions were common, emphasizing identifying and describing novel compounds rather than quantifying their concentrations. Chemists may now assess the reactivity of various substances based on the number of atoms or groups of atoms they can give to or absorb in a chemical reaction thanks to the notion of chemical equivalents, first presented in the middle of the nineteenth century. Friedrich Kohlrausch developed the concept of equivalents in the late 1800s, defining the equivalent conductivity of a solution as the conductivity of a solution with one equivalent of solute per litre of solution. The number of moles of solute per litre of solution is measured by molarity, a technique developed by chemists at about the same time to determine the concentration of solutions. Nevertheless, because it ignored the solute's number of equivalents, molarity was not always appropriate for defining the reactivity of compounds in solution. The idea of normalcy was first used to gauge solution concentration based on the number of solute equivalents in the early 1900s. Wilhelm Ostwald, a German chemist, coined the phrase "normal solution" in 1900 and described it as a solution having one gram of solute equivalent per litre of solution. The American scientist Julius Stieglitz first used the word "normality" to refer to this idea in 1904. Analytical chemistry immediately adopted the idea of normalcy to describe the reactivity of compounds in solution and figure out the stoichiometry of chemical processes. In acid-base titrations, normality was especially helpful since it allowed scientists to determine how many equivalents of an acid or base were required to neutralize a certain quantity of the other substance. The usage of normalcy began to wane towards the middle of the 20th century in favour of alternative concentration metrics like molarity and molality. Normality is still often utilized in analytical chemistry and some industrial applications, especially in creating electroplating solutions and other chemical processes where the reactivity of chemicals in solution is crucial. 
Normality DefinitionMolarity, or the number of moles of solute per litre of solution, is the foundation for normalcy. On the other hand, normalcy considers the number of reactive substances, such as hydrogen ions or electrons, in a solution.
Calculation of Normality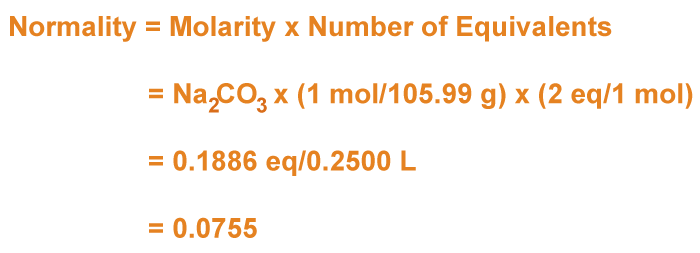
To calculate normalcy, finding the number of equivalents of a solute per litre of solution is necessary. A substance's equivalent is the volume that may react with one mole of hydrogen ions (H+). As a result, before calculating normalcy, one must ascertain how many equivalents of the solute are in the solution. The solute's chemical formula and the chemical process in which it is involved must be taken into account when calculating the number of equivalents. For instance, monoprotic hydrochloric acid (HCl) gives one H+ ion per molecule. As a result, one equivalent of H+ may be found in one mole of HCl. On the other hand, sulfuric acid (H2SO4) is a diprotic acid that contributes two H+ ions per molecule. Consequently, one mole of H2SO4 has two equivalents of H+. The normality of the solution may be calculated using the number of equivalents after it has been established. The quantity of the solute equivalents per litre determines a solution's normalcy. Hence, one must first ascertain how many moles of the solute is in the solution to compute normalcy. The molarity of the solution, or the number of moles of solute per litre, can be used to do this. For instance, a 1 M solution of HCl would have 1 mole of HCl in every litre of solution. The molarity must be multiplied by the number of equivalents to convert this to normalcy. There is only one equivalent per mole of HCl, so the normality is 1. The normalcy would be double the molarity for H2SO4, which has two equivalents per mole. For instance, a 1 M solution of H2SO4 would have a normalcy of 2 N and 2 equivalents of H+ per litre of the solution. It is essential to remember that a solution's normalcy depends on the reaction in which the solute takes part. As sodium carbonate (Na2CO3) may contribute two equivalents of OH- ions per molecule, it has a normalcy of 2 N when interacting with acids. Na2CO3 can behave as a weak acid when interacting with a base and only contribute one equivalent of an H+ ion per molecule, giving rise to the normalcy of 1. Normality is a concentration metric with various physical advantages in chemistry. The following are a few of the main physical advantages of normality:
Why "Normality" is a Better ChoiceWhen determining the concentration of a solution in chemistry, the idea of "normality" can be helpful, especially when dealing with acid-base interactions. The number of equivalents of a material per litre of solution is normal. A substance's equivalent is the quantity that may interact chemically with or take the place of one mole of hydrogen ions (H+) or hydroxide ions (OH-) in a process. The fact that normalcy considers the valence or charge of the species involved in the reaction is one benefit of employing it as a term in chemistry. For instance, a solution of sulfuric acid (H2SO4) and hydrochloric acid (HCl) may have the same molarity. Yet, because each sulfuric acid molecule may give two H+ ions, the sulfuric acid solution has twice the normalcy of the hydrochloric acid solution. Hydrochloric acid, on the other hand, can only give one molecule per molecule. Moreover, normalcy is more adaptable than other concentration metrics like molarity, molality, and per cent concentration. The nature of the reaction or the unique characteristics of the solution being measured is not considered by these parameters, even though they are helpful in and of themselves. For instance, utilizing the idea of "equivalents" rather than molarity or molality may be more applicable in a redox process. Normalcy also facilitates an understanding of stoichiometric calculations involving acid-base reactions. To be considered stoichiometric, an acid-base reaction has to have an equal amount of acid and base equivalents. Normalcy simplifies stoichiometric calculations by making it easier to determine how many equivalents of a certain material are involved in the reaction. Industrial Use of NormalityNormality is a crucial concept in industrial chemistry, where it is used in a wide range of applications. Some practical industrial uses of normality are:
ConclusionNormalcy is a helpful notion in chemistry because it considers the valence or charge of the species participating in a reaction, is more flexible than other concentration parameters, and is easier to apply in stoichiometric calculations involving acid-base reactions. Chemists can measure a solution's concentration and do calculations for chemical reactions more precisely by employing normalcy.
Next TopicOrganic Compound Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










