Organic Compound DefinitionOrganic substances are necessary for life to exist on earth. They are a large class of substances that are chemically linked in diverse ways between carbon and hydrogen atoms. The definition, characteristics, and relevance of organic compounds to daily life will all be covered in this article. 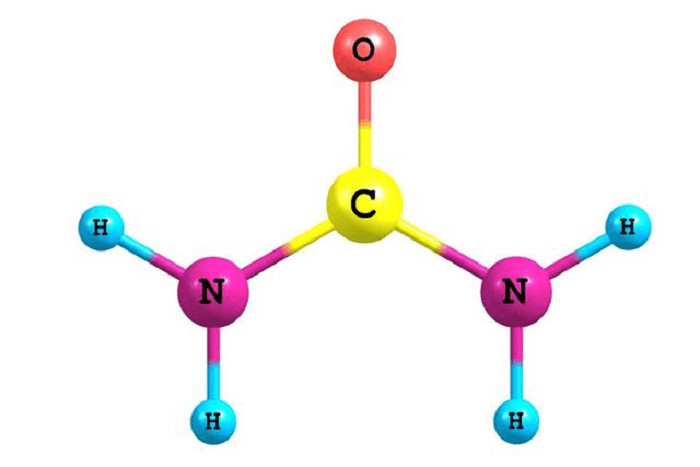
History Of Study Of Organic Compounds:The study of compounds has its origins in the early studies of chemists and alchemists who sought to comprehend the characteristics and behavior of natural substances. With the development of new methods for analysis and synthesis, the study of compounds evolved over time into one that was more systematic and scientific. The study of compounds has a particularly rich past when it comes to organic compounds, which are substances that include carbon and frequently hydrogen. Early chemists assumed that only organic molecules could be produced by living things and that these compounds were fundamentally distinct from inorganic compounds, which they thought could only be produced by natural processes. As chemists started experimenting with creating organic chemicals in the lab in the 19th century, this assumption was called into question. Friedrich Wöhler's 1828 experiment, one of the most well-known of these, involved creating urea, an organic substance present in urine, from inorganic components. This experiment helped eliminate the myth that organic substances were essentially different from inorganic ones and were a significant advance in understanding organic chemistry. Other well-known chemists, such as Justus von Liebig and August Kekulé, made significant contributions to the understanding of organic compounds over the course of the following several decades. The idea of chemical structure, which helped to explain the various characteristics and behaviors of organic compounds, is credited to Kekulé in particular. As the study of organic compounds advanced, scientists started to recognize and categorize many kinds of organic compounds based on their chemical makeup and physical characteristics. As an illustration, amines were originally discovered in the middle of the 19th century. These are organic compounds that include a nitrogen atom linked to one or more carbon atoms. When fresh analytical strategies and synthetic procedures were created in the 20th century, the study of organic molecules continued to advance. The mass spectrometer's creation, which allowed scientists to examine the structure and makeup of organic molecules in unprecedented detail, was one of the most important discoveries. Organic Compounds: Definition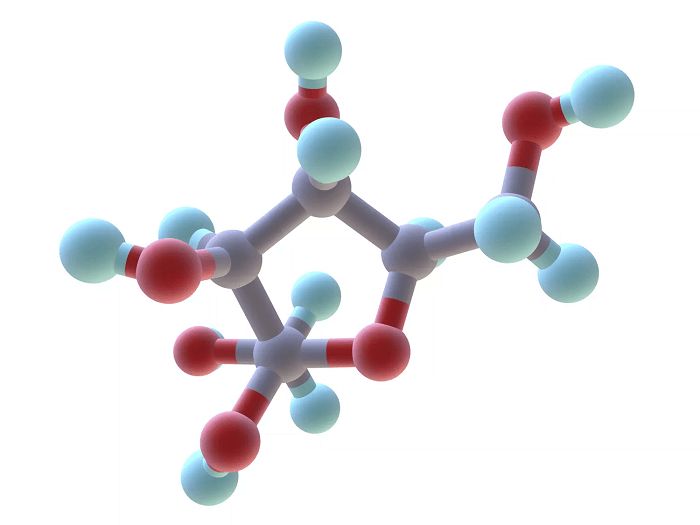
When biochemistry, which specialised in examining organic compounds in live creatures, was developed in the middle of the 20th century, the study of organic compounds experienced a significant alteration. The contribution that organic chemicals play in biological processes like metabolism and DNA replication has long piqued the curiosity of biochemists. As chemists and biochemists explore novel synthetic techniques, examine the characteristics of intricate organic structures, and create new uses for organic compounds in disciplines like medicine and materials science, the study of organic compounds is still an active area of research today. Living beings or their byproducts are referred to as "organic" in this context. Organic compounds in chemistry are molecules with carbon atoms bound to hydrogen atoms as well as occasionally additional elements including nitrogen, oxygen, sulfur, and halogens. In order to produce a stable structure, the carbon atoms in organic molecules join with other atoms in covalent bonds and share electrons. Organic compounds can be classified into two broad categories: natural and synthetic. 1. Natural Organic Compound Natural organic compounds are organic substances with carbon and frequently hydrogen. Several different types of natural sources, such as plants, animals, and microbes, contain these substances. Many organic substances found in nature serve crucial biological purposes and are necessary for life. For instance, proteins, lipids, and carbohydrates are all organic natural substances that are necessary for the existence of living things. Natural organic compounds serve a variety of utilitarian purposes in addition to their biological roles. Natural organic compounds are widely utilized as flavorings, perfumes, and colors. They are also crucial for the creation of novel treatments and pharmaceuticals. 2. Synthetic Organic Compound Chemical synthesis is a process used to create synthetic organic compounds in a lab. Usually, to produce these compounds, chemical building blocks, like carbon chains or rings, are combined, and the resulting compound is then altered to produce desired properties or functions. The usage of synthetic organic molecules is widespread in many different fields, including materials science, electronics, and medicine. For instance, many pharmaceuticals and medicines are made of synthetic organic molecules that are intended to treat certain diseases or biological processes. The creation of synthetic organic compounds has been essential to the creation of novel materials like polymers and plastics. These materials have a wide range of real-world uses, such as in the manufacture of consumer items and the building sector. The composition, characteristics, and behavior of chemical substances fall under two major categories: organic and inorganic. 1. Organic Compounds Organic substances are substances with carbon and frequently hydrogen and are generally present in living things. These may be basic molecules like methane or ethane, or they could be sophisticated compounds like proteins or DNA. Covalent bonds between carbon atoms are frequent in organic molecules, which also have lower melting and boiling temperatures than inorganic substances. 2. Inorganic Compounds Compounds that lack the connection between carbon and hydrogen are known as inorganic compounds. Simple molecules like water or salt or complex molecules like those found in minerals or metals might be present. Organic chemicals have lower melting and boiling temperatures than inorganic ones because they frequently contain ionic or metallic connections. The behaviour of organic and inorganic substances is another important distinction. In comparison to inorganic molecules, organic compounds are more reactive and subject to a larger spectrum of chemical reactions. This is due to the comparatively weak and readily breakable carbon-carbon and carbon-hydrogen bonds found in organic molecules. In comparison, inorganic substances are less reactive and more stable. These typical distinctions, however, are not universal. Examples of inorganic-organic hybrids include several inorganic substances that include carbon atoms, such as carbon dioxide and carbon monoxide. Similar to this, some organic substances, including carbonates and bicarbonates, are termed organic-inorganic hybrids because they lack hydrogen atoms. Properties of Organic CompoundsDue to their diverse molecular structures, organic compounds exhibit a wide range of physical and chemical properties. Some of the common properties of organic compounds include the following:
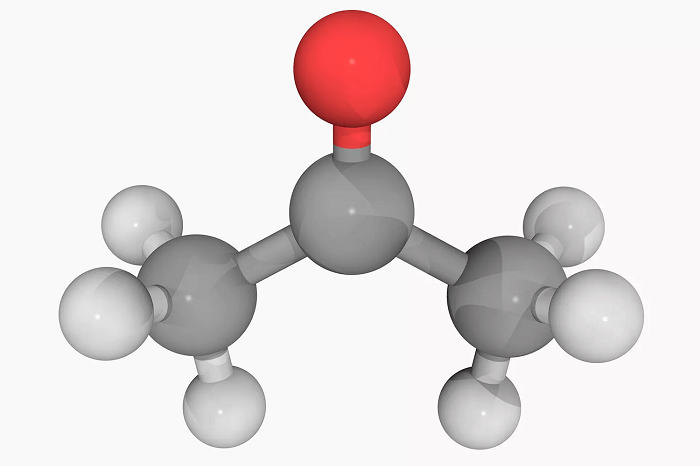
Importance of Organic Compounds:Organic compounds play a crucial role in many aspects of life, including:
Examples of Organic CompoundsOrganic compounds are ubiquitous and can be found in many forms in nature and industry. Here are some examples of organic compounds:
ConclusionEssential chemicals known as organic compounds play a crucial role in many facets of existence. They are distinguished by their distinct molecular architectures, many characteristics, and diverse uses. Several disciplines, including biology, agriculture, industry, and environmental research, depend on an understanding of organic molecules. By researching organic chemicals, scientists may create new goods, advance existing technology, and solve urgent global problems.
Next TopicOrganization Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










