Organic CompoundsA compound can be any substance made of two or more elements. The elements are chemically bonded to each other in a compound. A compound can be of two types: organic and inorganic. What is an organic compound?In general, the compounds that contain carbon and hydrogen atoms are called organic compounds. They are made of molecules that contain carbon-carbon covalent bonds (c-c, c=c, c---c) or carbon-hydrogen (c-h) covalent bonds or both types of bonds. For example, C2H6 (CH3-CH3) is an organic compound as it contains both carbon-carbon and carbon-hydrogen covalent bonds. Similarly, CH4 (methane) is an organic compound. Carbon dioxide is not an organic compound as it does not contain carbon-carbon or carbon-hydrogen bonds. Similarly, sulphuric acid is not an organic compound. Organic compounds are large molecules as they are made of chains of carbon and hydrogen atoms attached to various other atoms. For example, CH3-OH (methyl alcohol or methanol) and CH3-NH2 (Methylamine). Organic compounds are called organic because they were once believed to be derived from living things or are associated with living things. A large number of organic compounds are found naturally, however, they can be synthesized. Some common examples of organic compounds are fats, carbohydrates, proteins, nucleic acids, petroleum, natural gas, and more. These molecules are studied in detail in biochemistry or organic chemistry disciplines of organic chemistry. The main types of organic compounds found in living organisms are carbohydrates, lipids, proteins and nucleic acids. These are described below one by one; 1. CarbohydratesThese organic compounds are made of carbon, hydrogen and oxygen elements. The ratio of hydrogen and oxygen atoms in carbohydrates is 2:1. They act as energy sources and structural units for living organisms. They are the most abundant organic compounds found in living organisms. Some common examples of carbohydrates are glucose, fructose, sucrose, chitin, and cellulose. A carbohydrate can be made of one or more types of unit structures (monosaccharides) accordingly they can be of the following types; Monosaccharide: This type of carbohydrate is made of a single unit structure or only one saccharide unit. They are monomers of carbohydrates as carbohydrates are made of monosaccharides. They cannot be hydrolyzed into simpler forms. Its common examples are glucose, fructose, and galactose. They are sweet in taste, water-soluble and a source of energy for the human body. Disaccharide: It is made of two unit structures or two monosaccharides joined together. They can be hydrolyzed into simpler forms. For example, Sucrose, a disaccharide is made of glucose and fructose. Similarly, lactose (disaccharide) is made of glucose and galactose. Oligosaccharides: They are made of more than two saccharide units or monosaccharides. Polysaccharides: They are made of many (more than ten) unit structures (monosaccharides) of carbohydrates. They are not sweet in taste, insoluble in water and act as storage or structural molecules. 2. LipidsLipids are also organic compounds made of carbon, hydrogen and oxygen atoms. The ratio of hydrogen and oxygen atoms is higher in lipids than in carbohydrates. There are three main groups of lipids which are as follows; Triglycerides: They are fat that we get from food and is carried in the blood. We eat most of the fat in the form of triglycerides. The excess calories in our body are also converted into triglycerides. They are made of three fatty acids bonded to a glycerol molecule. Common examples include fats, oils and waxes. Steroids: This organic compound contains four carbon rings joined to each other and arranged in a specific molecular configuration. This molecular configuration or fused ring structure contains 17 carbon atoms. The most common example of steroid is cholesterol, which acts as a precursor to vitamin D, testosterone, estrogen, cortisol, bile salts, and more. Phospholipids: This type or class of lipids are made of molecules that contain hydrophilic ‘head’ with a phosphate group and two hydrophobic chains made of fatty acids, so also known as fatty acid chains. Phospholipids resemble triglycerides with only one difference that in triglycerides one of the fatty acid chains is replaced by a phosphate group. 3. ProteinsProteins are also organic compounds. They are made of amino acids called peptides. They can be made of a single polypeptide chain or more polypeptides chains that are packed together to form a unit. Proteins are generally made of atoms of hydrogen, carbon, oxygen, and nitrogen. However, they may contain atoms of other elements such as iron, copper, Sulphur, magnesium, phosphorus, etc. These organic molecules play vital functions in cells such as they are building blocks of structures, act as a catalyst in biochemical reactions, are needed for immune responses, needed to pack and transport material and also assist the replication of DNA. Some common examples of proteins include enzymes, keratin, hemoglobin, fibrin, albumin, and more. 4. Nucleic AcidsThese organic compounds are made of nucleotide monomer chains. A nucleotide is made of a nitrogenous base, sugar molecule and phosphate group. Nucleic acids are responsible for the transmission of genetic information from one generation to another generation. Common examples of nucleic acids include DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). Besides the above four types of organic molecules that are found in organisms, there are also other types of organic molecules such as drugs, vitamins, dyes, toxins, and more. Classification of Organic CompoundsThe organic compounds can be classified in two different ways; based on structure and based on the functional group. Based on structure, the organic compounds can be of the following two types:
1. Acyclic or open chain compoundsAs the name suggests, they have a linear structure and don’t form any ring. They may have branched or straight open chains of carbon atoms in their molecules. So, their terminal carbon atoms are completely free or not linked with each other. They are also known as aliphatic compounds. For example; Propane (C3H8) or CH3-CH2-CH3 In the above arrangement of atoms of carbon and hydrogen in propane, we can see that terminal carbons are not bonded with each other, however, they are linked with hydrogen atoms. Similarly, in pentane (C5H12) the terminal carbon atoms are not linked with each other rather with hydrogen atoms. Similarly, acetic acid (CH3-COOH) is an open chain compound. 2. Cyclic or closed-chain compoundsIn this type, the carbon atoms are connected to each other to form closed ring like structures. So, these compounds have one or more closed chains or rings of atoms in their molecules. This is because the terminal carbon atoms are bonded or linked with each other which results in a closed ring like structure. The rings in a compound need not be of the same size. Cyclic organic compounds are further divided into two types; heterocyclic and homocyclic. i) Heterocyclic:In this type of cyclic organic compound, the ring structure is made of atoms of two or more different elements. One type of atoms are of carbon and but the other types of atoms are always of different elements such as nitrogen, oxygen, Sulphur, etc. For example, synthetic dyes, nucleic acids, drugs, etc. They are also divided further into two types: Alicyclic and Aromatic heterocyclic compounds. a) Alicyclic heterocyclic compounds These are the compounds that contain one or more heteroatoms (atoms other than carbon or hydrogen) in their rings. For example, tetrahydrofuran, tetrahydrothiophene, tetrahydropyrole, and more. 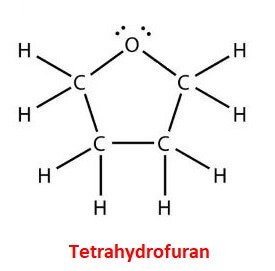
In the above image, we can see the heteroatom oxygen in tetrahydrofuran. b) Aromatic heterocyclic compounds They contain one or more heteroatoms in their molecules and are aromatic in nature. For example, furan, pyrrole, thiophene, etc. 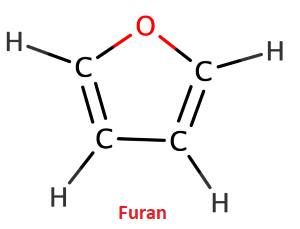
ii) HomocyclicIn this type of cyclic compound, the ring is made of the same type of atoms or atoms of only one element which is always carbon. So, they are also called carbocyclic compounds as no other element can form this type of compound. For example, tetracene, benzene, naphthalene, and more. However, in inorganic chemistry, there are Homocyclic compounds with ring structures made of atoms of different elements such as sulphur, boron, phosphorus, and more. Homocyclic or carbocyclic compounds are further divided into two following two types; a) Alicyclic compound: This type of homocyclic compound is aliphatic as well as cyclic. It has one or more rings made of carbon atoms. These carbon rings can be saturated or unsaturated, which means bonds between two atoms can be single, double or triple covalent bonds. These compounds resemble aliphatic (closed ring) compounds in most of their properties. So they are called alicyclic which means cyclic compounds resembling aliphatic compounds. For example; Cyclopropane (C3H6) 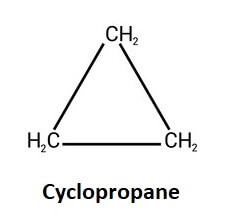
Similarly, cyclobutane and cyclohexane are alicyclic compounds. b) Aromatic Compounds: This type of Homocyclic or carbocyclic compound contains alternate double and single bonds between carbon atoms. For example, benzene as shown below; 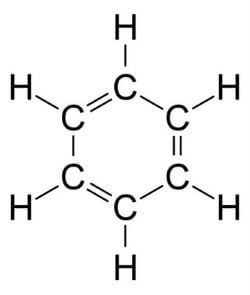
In the structure of benzene above we can see the alternate single and double bond between carbon atoms. Aromatic carbocyclic compounds are further divided into benzenoid aromatic compounds and non-benzenoid aromatic compounds. i) Benzenoid Aromatic Compounds As the name suggests, these aromatic carbocyclic compounds contain one or more fused benzene rings. For example, benzene has one ring as shown above, and naphthalene has two fused benzene rings and anthracene is made of three fused benzene rings as shown below; 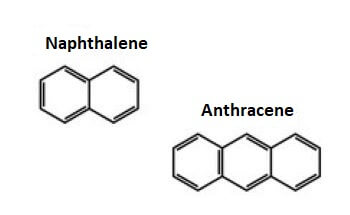
ii) Non-benzenoid Aromatic Compound These aromatic carbocyclic compounds do not contain a benzene ring. For example, azulene (C10H8) as shown below; 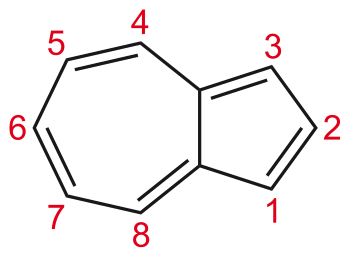
Classification of organic compounds based on functional groupA functional group can be an atom or a group of atoms that replaces the hydrogen atom in an organic compound. The addition of functional group changes the chemical properties of the compound in which it replaces the hydrogen atom. The functional group gives the compound its characteristic chemical properties. The organic compounds with the same functional group belong to the same class of organic compounds. For example, the hydrogen atom of methane when replaced by hydroxyl group (- OH), it is converted into methanol, which is a type of alcohol that belongs to the alcohol class of organic compounds. Based on the functional group, some of the major classes in which organic compounds can be classified are given below:
Next TopicAcetone
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










