Osmosis DefinitionThe process whereby solvent molecules move from a solution that is lower in concentration to one that has a higher concentration via the semipermeable membrane is known as Osmosis. It's a passive process that occurs without the expense of energy. The transportation of solvent molecules goes on between high and low-concentration areas until the concentration of each end of the membranes is the same. In 1877, Wilhelm Pfeffer, a German plant physiologist, first examined this process thoroughly. Previous studies of the leaky membranes (e.g., animal bladders), as well as the flow of chemicals and water through them, were less specific. A British scientist named Thomas Graham coined the term "osmose" (now called Osmosis) around 1854. If the liquid is separated from pure solvent by a membrane permeable to the solvent but not to the solute, the solution is likely to become more dilute because the solvent is absorbed by the membrane. By increasing the pressure of the liquid by a specific amount, this process could be slowed. In 1886, Jacobus Van 't Hoff, a Dutch-born chemist, proved that if the solute is so weak that its vapor pressure in the solution is in accordance with Henry's Law (i.e., it is proportional to the amount of it within the solution) and then, it is possible to alter the osmotic pressure according to temperature and concentration in a way similar to the gas that occupies similar volumes. This resulted in formulae to estimate the molecular masses of solutes in dilute solutions based on the temperature, freezing, and vapor pressure. 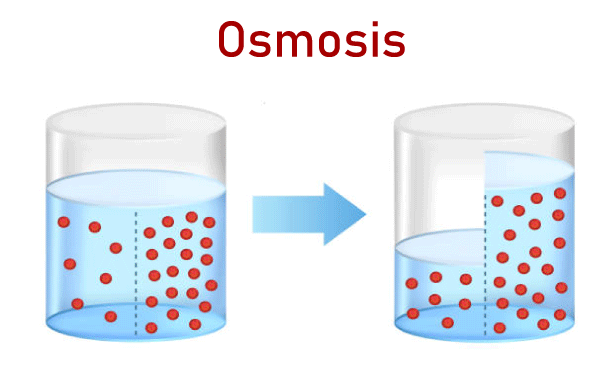
Osmotic PressureThe Osmotic pressure is the minimum pressure that is required to block the flow of an inward-facing solution's solvent that is pure through a semipermeable barrier. It could also be described as the measure of a solution's capacity to absorb pure solvent by Osmosis. The most powerful osmotic pressure that can develop in a solution if it is removed from the solvent it is derived from by the semipermeable membrane is referred to as the potential osmotic pressure. If two solutions with different amounts of solvent are separated through a membrane that is selectively permeable, there is Osmosis. From the solution with a low concentration to the solution that has a higher solute content, the solvent molecules move across the membrane more frequently. The motion of solvent molecules will continue until equilibrium has been reached. What's a Semipermeable Membrane?It's a thin layer between two solutions, which allows certain elements of the solution, typically the solvent, to flow through. A semipermeable membrane acts as one that allows specific molecules to cross but stops other molecules from crossing. The semipermeable membrane acts as a filter. Semipermeable membranes of all kinds are able to block molecules of different dimensions. Chemical or synthetic substances can be used to make semipermeable membranes. A partially permeable or a deferentially permeable are different names for semipermeable membranes. Different types of OsmosisIn general, there are two kinds of Osmosis- 1. EndosmosisWhen a cell is placed in a hypotonic solution, water circulates within the cell, causing it to grow or plasmolyze. This occurs because the concentration of solute in the solution is lower than that of the cell. This process is referred to as endosmosis. The process of Osmosis toward the inside of a vessel or cell is referred to as endosmosis. It occurs when the potential of water outside the cell is higher than the one within the cell. In the end, the solute concentration in the surrounding solution is lower than that in the cell's cytoplasm. Hypotonic solution is the proper name for this kind of solution. In the process of endosmosis, water molecules travel over the cell's membrane and inside the cell. The entry of water molecules inside cells triggers them to expand. Example: When placed in normal water, raisins tend to swell. 2. ExosmosisWhen a cell is placed in a hypertonic solution, the water within the cell escapes and, consequently, the cell undergoes plasmolysis (becomes in a flaccid state). This occurs because the concentration of solute in the solution is higher than the concentration in the cell's cytoplasm. This is known as exosmosis. Exosmosis refers to the osmosis process that moves the vessel or cell toward the outside. It occurs when the potential of water within the cell's environment is less than the water potential within the cell. In the end, the solute concentration in the surrounding solution is greater than that of the cells' cytoplasm. These types of solutions are referred to as hypertonic solutions. Exosmosis refers to the movement of water molecules from cells across cells' membranes. The movement of water molecules out of cells causes cells to shrink. Example: When placed in a salty solution, raisins tend to shrink. Reverse OsmosisIt is a separate procedure that makes use of pressure to push a solvent through an impermeable membrane that holds the solvent on one side and allows the solvent to pass across the opposite side. The process uses pressure to make the solvent be moved from a high concentration zone to a lower concentration zone. This is why reverse Osmosis could be described as the reverse of general Osmosis. Forward OsmosisThis is an organic process that utilizes a semipermeable membrane to remove dissolved substances from water. The forward osmosis method is beneficial in a wide range of water treatment processes in the industrial sector, such as water treatment as well as product concentration and water recycling, because of its highly efficient filtering process, which guarantees the purest water gets recovered out of the solution feed. It consumes lesser energy than other pressure-based hydraulic water treatment systems because it is based on the natural energy that comes from the osmotic pressure. Osmotic ConcentrationThe osmolarity of a solution can be an indication of how concentrated the substance is within a liter of solution. Osmoles (Osm) are used to determine the osmotic concentration that is expressed as the number of osmoles in a liter (Osm/L). In certain instances, the Osmotic concentration can also be measured by millimoles per liter (mmol/L). The osmotic content of the solvent increases when the quantity of solvent or water diminishes. Similarly, to that, increasing the amount of liquid in the solution lowers the osmotic concentration of the solvent. Isotonic SolutionIn an isotonic liquid, cells are at equilibrium with its surrounding when the levels of substances inside and outside are equal (iso refers to equal is the Greek word for equal in Latin). There isn't any concentration gradient within this condition, meaning that there isn't any significant movement of water in or out. But water molecules are able to move into and out of the cell at the same time across both directions. Hypotonic SolutionThe solute concentration in a hypotonic liquid is lower than within the cells (the prefix hypo is Latin for below or under). The cell is flooded with water due to the differences in concentration across the various compartments. Animal cells are less intolerant of this situation than plant cells. Water is pumped into the intercellular space when the massive central vacuole gets filled with water in the plant. The pressure of Turgor is caused due to the combination of two processes that push against the cell's wall, which causes it to expand. Cell walls act as a wall to stop the cell from expanding. A cell from an animal, however, expands until it explodes and dies when placed in a hypertonic solution. Hypertonic SolutionThe prefix hyper means "above" in Latin. The number of solutes present in hypertonic fluids is greater than within the cell. The water rushes out, which causes cells to shrink or wrinkle. This is evident within red blood cells, which undergo a process known as crenation. Because of the processes that take place inside, the plant cells within hypertonic solutions may resemble the shape of a pincushion. The cell membrane dries away from the cell's wall; however, at plasmodesmata, it remains attached. Plasmodesmata are tubes of small size that carry and transmit information between plant cells. Plasmolysis happens when the inner membrane contracts, which causes the plasmodesmata's walls to contract. Osmosis in a plant cell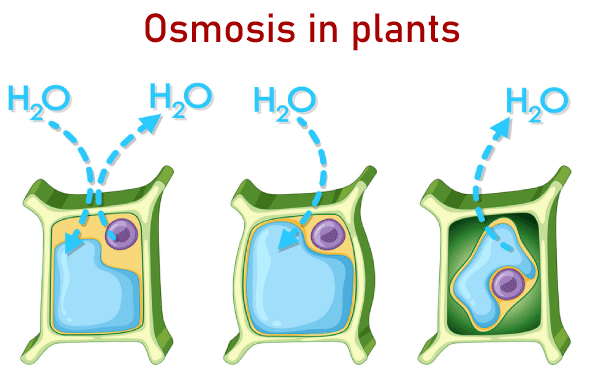
In the event that a cell is placed in a solution with hypertonic elements that is more concentrated than the cell, it will shrink as a result of loss of water and then cease to exist. For instance, If a small bit of carrot is placed in a salty solution of water, it will turn limp and soft because the cells will shrink. However, in contrast to this, if the carrot is placed in an isotonic solution, it will expand and swell. A normal cell will explode; however, the strong wall of the cell keeps it from rupturing. When water is introduced inside the cells, it increases until it puts a high pressure on the cell's walls to expand even more. However, the cell's wall expands again with the same pressure, and no more water can get in. Osmosis is an essential factor in the movement of water within plants. The concentration of solutes increases when they travel from the soil to root cells and finally to leaf cells. The pressure difference helps to push the water up. Osmosis also helps to control the loss of water from leaves by controlling the size of the stomata located on the leaf's surface. The significance of Osmosis:In the plants:
In Animals:
The overall significance of Osmosis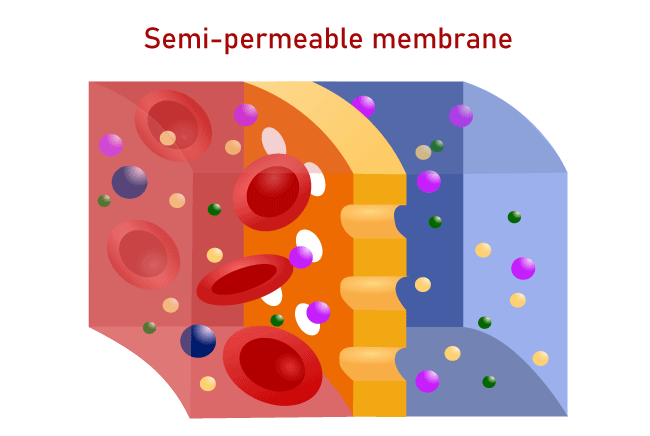
Examples of Osmosis
What is Diffusion?Diffusion is caused by the random movement of molecules. It results in an overall transfer of matter from a high-concentration zone to a low-concentration. One common example is the smell of a flower that quickly diffuses throughout a room's air. In the case of fluids, heat conduction is the process of transfer of thermal energy from a greater temperature to a lower temperature. Nuclear reactors require the diffusion of neutrons in the material, which often scatters the neutrons, but rarely absorbs them. Examples of Diffusion
Next TopicHealth Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










