Osmosis Definition BiologyOsmosis is a fundamental biological mechanism essential to the health of living creatures' cells and tissues. It describes the migration of water molecules from a region of high water concentration (low solute concentration) to a region of low water concentration through a semi-permeable barrier (high solute concentration). The natural propensity of water to flow from places of high concentration to areas of low concentration to reach equilibrium is what propels the process. 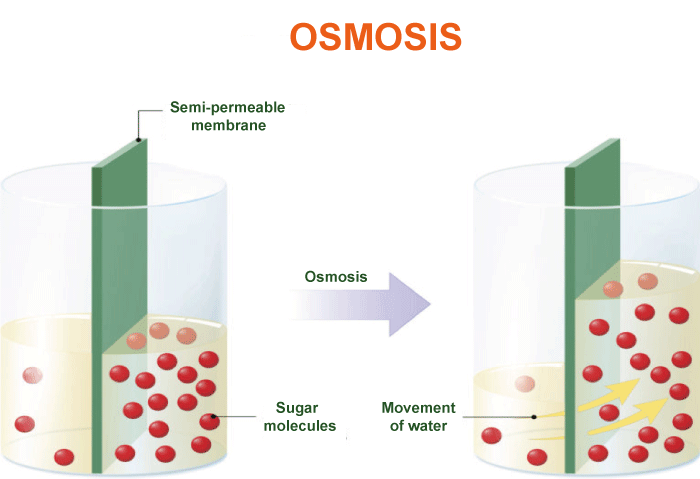
Background StudyThe French biologist René Joachim Henri Dutrochet initially proposed the idea of osmosis in 1826 after noticing that plant cells will either expand or shrink depending on the concentration of a solution containing salt, sugar, or other solutes. Thomas Graham, a British scientist, first used the term "osmosis" in 1854 to refer to the "passage of liquids through animal or vegetable membranes." Domain Of StudyOsmosis is a fundamental biological and biochemical concept that is investigated in a number of disciplines, including cell biology, physiology, and biophysics. Osmosis is fundamentally the transfer of water molecules from a region of high concentration to a region of low concentration across a membrane that is selectively permeable. This process starts on its own without any external energy. Understanding the characteristics of the membrane that divides the two solutions from one another as well as the characteristics of the solutions themselves is essential to the study of osmosis. For instance, the membrane has to be selectively permeable, which means that only some molecules may get through while others are blocked. In order to provide a concentration gradient that propels the passage of water, the solutions on each side of the membrane must also contain various concentrations of solutes, such as salts or sugars. Osmosis plays a crucial function in fundamental biology and also has several practical uses. For instance, osmosis is essential to human cellular function, and knowledge of its mechanics can aid in the development of novel therapeutic approaches for disorders that impair cellular function. Osmosis is also employed in several industrial procedures, including the desalination of saltwater and the creation of certain foods and drinks. Many experimental methods, like as microscopy, electrophysiology, and mathematical modelling, are used to examine osmosis. These methods may be used by researchers to look at issues including how osmosis is impacted by various membrane types, how changes in temperature or pressure affect osmosis, and how osmosis can be controlled for usage in real-world scenarios. 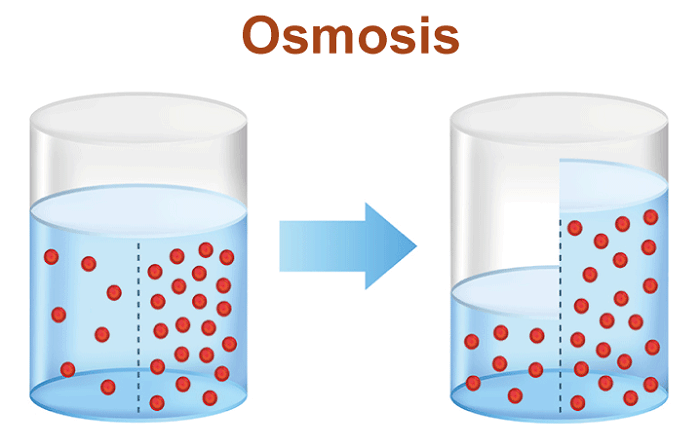
The Process Of OsmosisThis process is crucial for the survival of living organisms, as it regulates water balance within cells and tissues. The process of osmosis can be broken down into several key steps. First, there must be a difference in the concentration of solutes (particles dissolved in water) on either side of the membrane. This difference creates what is known as an osmotic gradient, which drives the movement of water molecules. When the solute concentration is higher on one side of the membrane, water molecules will move from the side with a lower solute concentration to the side with a higher solute concentration to equalize the concentration on either side of the membrane. As water molecules move across the membrane, they interact with the solute molecules, causing them to become diluted. This dilution of the solute molecules reduces the overall concentration of solutes on the side of the membrane that was originally more concentrated while increasing the concentration on the side that was originally less concentrated. This process continues until the concentration of solutes on both sides of the membrane is equalized, at which point the movement of water molecules stops. The pressure required to stop this movement of water molecules is known as the osmotic pressure. One important aspect of osmosis is that it only allows for water molecules' movement, not solute molecules. This means that if a solute molecule is too large to pass through the membrane, it will not be able to move from one side of the membrane to the other, even if there is a difference in solute concentration. CharacteristicsIt's essential first to comprehend the characteristics of a semi-permeable membrane in order to comprehend osmosis. A membrane that permits certain molecules or ions to flow through while obstructing others is a semi-permeable membrane. Cell membranes are an example of a semipermeable membrane in biological systems because they permit the passage of water molecules, tiny molecules like oxygen and carbon dioxide, and certain ions while obstructing the passage of bigger molecules and the majority of ions. The difference in solute concentration on each side of a semi-permeable membrane causes the migration of water molecules across it. Like salt in water, particles called solutes are dissolved in a liquid. Osmotic pressure, a measurement of the propensity of water to migrate across a membrane in response to variations in solute concentration, may be used to determine the concentration of solutes in a solution. Water molecules will cross a semi-permeable membrane separating pure water from a solution in order to balance the concentration of solutes on each side of the membrane by moving from the side with lower solute concentration to the side with greater solute concentration. The osmosis process is what causes this. Many biological situations allow for the observation of osmosis. Osmosis, for instance, is essential to plant cells' shape and turgor pressure. A plant cell will enlarge and become turgid when submerged in a hypotonic solution, a solution with a lower concentration of solutes than the cell. In contrast, water molecules will flow out of a plant cell when put in a hypertonic solution (a solution with a greater concentration of solutes than the cell), causing the cell to contract and lose turgor pressure. Animal cells' ability to operate depends on osmosis as well. For instance, red blood cells depend on osmosis to keep their structure and functionality. Red blood cell membranes are semi-permeable, allowing water molecules to flow through but blocking bigger molecules like proteins. A red blood cell may expand and perhaps rupture when put in a hypotonic solution because water molecules will flow inside the cell. A red blood cell will shrink and may sustain damage if put in a hypertonic solution because water molecules will leave the cell when this happens. Osmosis plays a significant role in several industrial processes, including the desalination of saltwater and the filtration of drinking water. Reverse osmosis is frequently used in water treatment to eliminate impurities. In this procedure, pressure is used to push water through a semi-permeable membrane, allowing water molecules to pass through but blocking contaminants like bacteria, viruses, and dissolved minerals. After that, the cleaned water can be consumed or utilized for various things. Osmosis is a normal and significant function, but it can also be harmful under some circumstances. For instance, excessive osmosis can result in the organism being dehydrated because water molecules are transferred from cells to the circulation to balance the concentration of solutes. This can happen when there is a large concentration of solutes in the bowel, as occurs in conditions like diarrhea. Osmosis can further contribute to the rotting of some items, including fruits and vegetables. Water molecules may enter the food cells of these meals when they are kept in a high-humidity environment, causing the food cells to enlarge and become mushy or squishy. 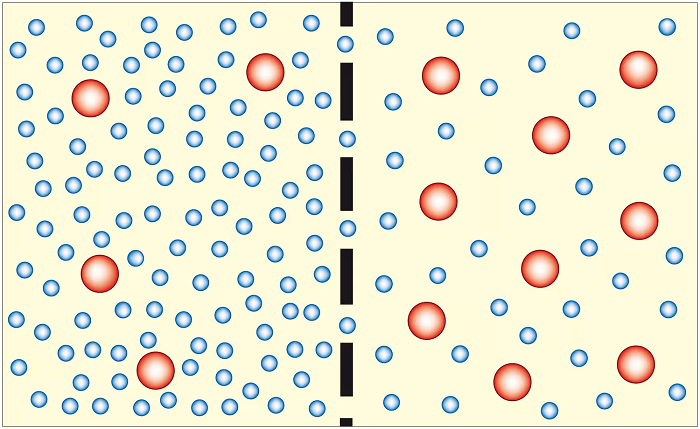
Practical Applications Of OsmosisThe process of osmosis has a wide range of practical uses in various fields. Some of the most common applications of osmosis include water purification, food preservation, and medical treatments. One of the most important uses of osmosis is in water purification systems. Reverse osmosis (RO) is a process that uses a semi-permeable membrane to remove impurities and contaminants from water. The membrane allows only water molecules to pass through while blocking larger particles, such as bacteria, viruses, and minerals. RO systems are used in households and industries to provide safe and clean drinking water. Another practical application of osmosis is in food preservation. When food is placed in a solution of high concentration, the water inside the food cells moves out, causing the cells to shrink. This process is called plasmolysis and can help preserve food by reducing the growth of microorganisms and preventing spoilage. This technique is commonly used in the production of dried fruits, vegetables, and meat products. In medical treatments, osmosis is used to control the fluid balance in the body. For instance, in cases of dehydration, an intravenous (IV) solution of saline is administered to the patient. The saline solution has a higher concentration of salt than the cells in the body, which creates an osmotic gradient that draws water from the cells into the bloodstream. This helps to restore the fluid balance in the body. In real life, osmosis also affects human health in various ways. For instance, the kidneys play a critical role in regulating the water balance in the body through osmosis. They filter the blood and remove excess water and waste products while retaining essential nutrients and ions. However, certain medical conditions, such as diabetes and kidney diseases, can affect the osmotic balance in the body, leading to complications such as dehydration or overhydration. In these cases, medical interventions such as IV therapy or dialysis are necessary to restore the proper fluid balance. The process of osmosis is an important topic taught in various academic fields, such as biology, chemistry, and environmental science. There are several ways and experiments used to teach this process to students. One of the most common ways to teach osmosis is through visual aids such as diagrams, animations, and videos. These aids help to illustrate the concept of osmosis and its various applications in real-life scenarios. Another effective method to teach osmosis is through laboratory experiments. Students can perform experiments such as the potato osmosis experiment, which involves placing a potato in solutions of varying salt concentrations to observe the effects of osmosis on the potato cells. Similarly, students can also perform the egg osmosis experiment, which involves placing an egg in vinegar to dissolve the shell and then placing it in a solution of varying concentrations to observe the effects of osmosis on the egg membrane. In addition, osmosis can also be taught through simulations and computer models. These tools help students to visualize the process of osmosis at the molecular level and understand how the concentration gradient affects the direction of water movement. ConclusionIn conclusion, the function of cells and tissues in living creatures fundamentally depends on the osmosis process. It is crucial for maintaining correct cell shape and function and includes the passage of water molecules across a semi-permeable membrane in response to variations in solute concentration. Osmosis is a vital and natural mechanism, but it may also backfire and cause dehydration and food spoiling.
Next TopicPathology Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










