Osmotic Pressure DefinitionIntroductionOsmotic pressure is the pressure exerted by the movement of water molecules through a semipermeable membrane from a lower solute concentration region to a higher solute concentration region. It is a colligative property that depends on the number of solute particles in a solution rather than the type. The greater the solute particles in a solution, the higher the osmotic pressure. What is Osmotic Pressure?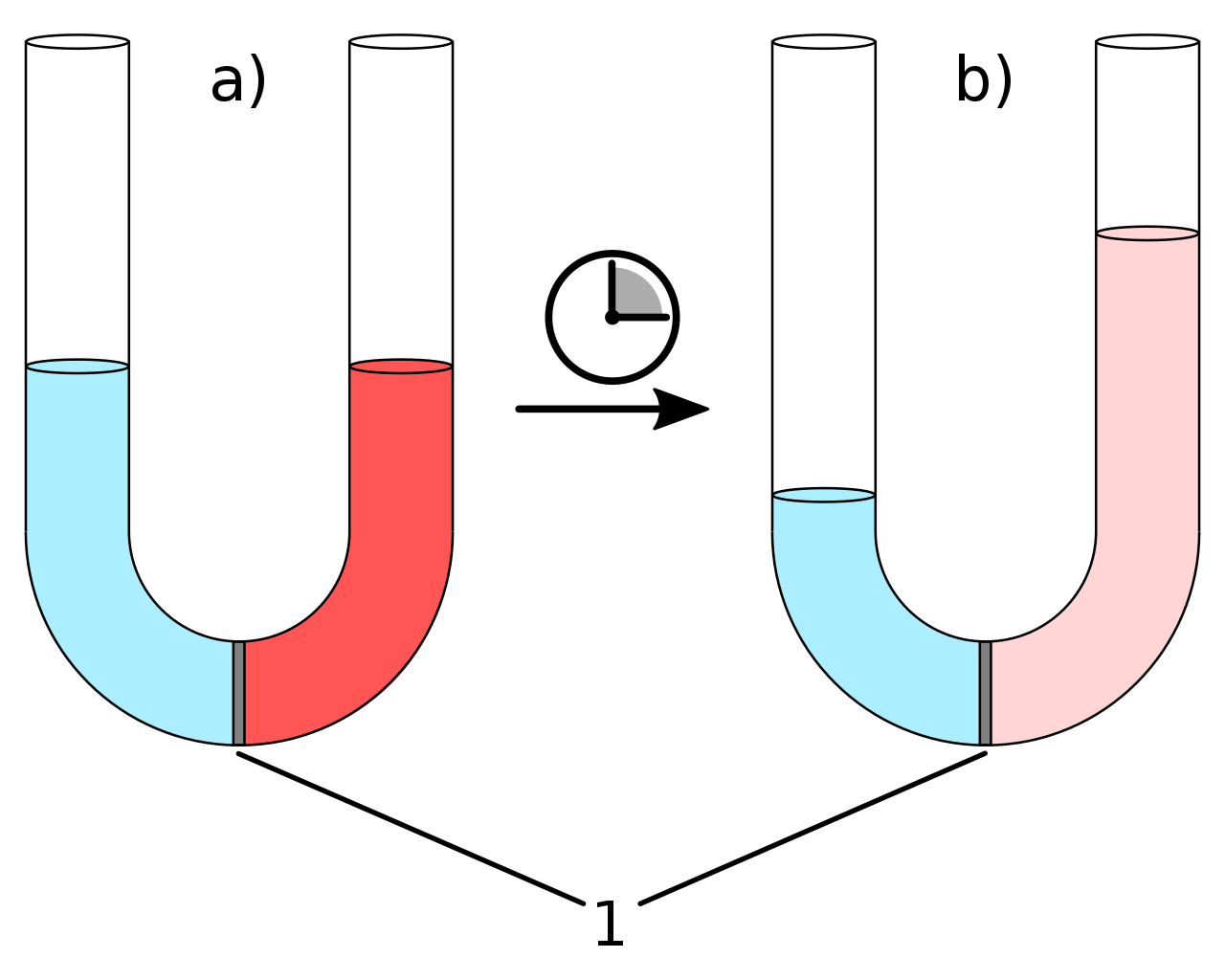
Osmotic pressure is the pressure required to prevent the flow of water molecules through a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration. This pressure is created by moving water molecules from the side of the membrane with a lower solute concentration to the side with a higher solute concentration to equalize the solute concentration on both sides of the membrane. Osmotic pressure is a fundamental concept in many biological and industrial processes. It plays a critical role in maintaining the water balance of cells and organisms, as well as in water purification and the production of certain industrial products such as pharmaceuticals and food products. Osmotic Pressure EquationThe osmotic pressure equation is given by: π = iMRT where:
This equation shows that osmotic pressure is directly proportional to the concentration of solute particles and the absolute temperature and is inversely proportional to the volume of the solution. It also demonstrates that osmotic pressure is a colligative property that depends on the number of solute particles in the solution and not on the identity of the solute particles. Osmotic Pressure ExampleHere is an example of how to calculate osmotic pressure: Suppose we have a solution of glucose (C6H12O6) at a concentration of 0.1 M. We want to calculate the osmotic pressure at room temperature (25°C or 298 K). First, we must find glucose's van't Hoff factor (i). Since glucose does not dissociate in solution, its van't Hoff factor is 1. Next, we can plug the values into the osmotic pressure equation: π = iMRT π = (1)(0.1)(0.08206)(298) π = 2.43 atm Therefore, the osmotic pressure of the glucose solution at room temperature is 2.43 atm. This means that if the glucose solution were separated from pure water by a semipermeable membrane, water would flow from the pure water side to the glucose solution side until the pressure on the glucose side reached 2.43 atm. The concentrations of solutes on both sides of the membrane were equalized. Applications of Osmotic PressureOsmotic pressure has many applications in various fields, such as biology, medicine, and industry. Some of the most important applications are:
Types of Osmotic PressureOsmotic pressure may be broadly divided into three categories based on the solute and solvent components:
Next TopicParallelogram Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










