Ozone Layer DefinitionA portion of Earth's stratosphere known as the ozone layer has a significant concentration of ozone molecules (O3). This layer serves as a screen from the sun's harmful ultraviolet (UV) radiation and is situated about 10-50 km (6-30 miles) above the surface of the Earth. Ozone molecules are formed naturally in the stratosphere when oxygen molecules (O2) are split apart by high-energy UV radiation. The liberated oxygen atoms that are left behind can combine with other oxygen molecules to create ozone. Most of the UV radiation from the sun is absorbed by ozone, which prevents it from reaching the surface of the Earth, where it may harm plants, animals, and humans by causing skin cancer, cataracts, and other health issues. In recent decades, there has been concern about the depletion of the ozone layer due to the release of human-made chemicals called chlorofluorocarbons (CFCs), which can break down ozone molecules. The Montreal Protocol, an international treaty signed in 1987, has helped to limit the use of CFCs and other ozone-depleting substances, allowing the ozone layer to recover slowly over time. 
How Is The Ozone Layer Formed?By a series of intricate chemical reactions combining ultraviolet (UV) light from the sun and oxygen molecules (O2) in the atmosphere, the ozone layer is naturally created in the Earth's stratosphere. This is a quick synopsis of the procedure:
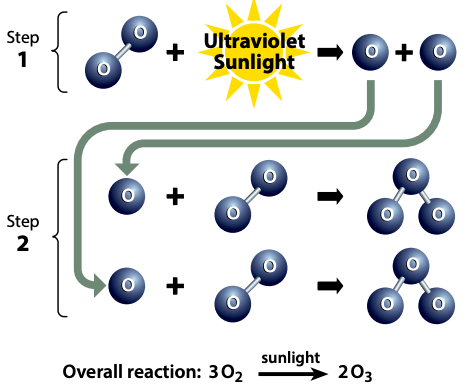
Overall, the formation and maintenance of the ozone layer is a delicate balance between the natural production and destruction of ozone molecules. This equilibrium can be upset by human actions and result in the ozone layer being depleted, such as the discharge of specific chemicals into the atmosphere. What Is Ozone Depletion?Ozone depletion refers to the loss of ozone molecules in the Earth's stratosphere, particularly in the ozone layer. This is a problem since ozone is essential for shielding the planet from the sun's harmful ultraviolet (UV) radiation. The atmospheric release of man-made compounds termed chlorofluorocarbons (CFCs) is the main factor contributing to ozone depletion. 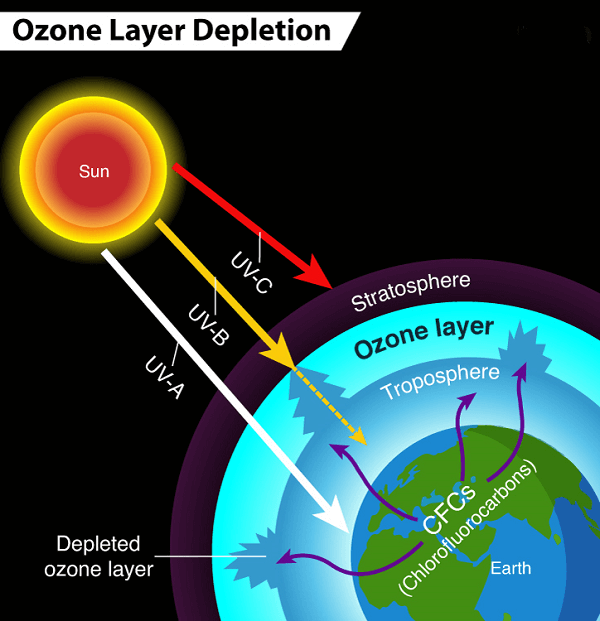
CFCs were commonly used as refrigerants, solvents, and propellants in aerosol sprays. After being released into the atmosphere, they ascend to the stratosphere where UV light breaks them down. The resulting chlorine atoms (Cl) can then react with ozone molecules (O3), breaking them apart and releasing oxygen molecules (O2). This reaction is known as the ozone depletion reaction and it depletes the ozone layer. The economy, the environment, and human health could all suffer significantly from the ozone layer's loss. Those who are exposed to UV light more frequently may develop cataracts, skin cancer, and other health issues. It can also damage crops and aquatic ecosystems, affecting food production and biodiversity. In addition, ozone depletion can lead to changes in the Earth's climate by altering atmospheric temperatures and circulation patterns. In 1987, the Montreal Protocol, an international agreement, was signed to address the problem of ozone depletion. The protocol mandated the phase-out of ozone-depleting substance manufacturing and consumption, including CFCs. The ozone layer has started to slowly recover as a result of the protocol's success in lowering the concentrations of ozone-depleting compounds in the atmosphere. The ozone layer will, however, take several decades to fully regenerate. To guarantee the ozone layer's protection in the future, ongoing initiatives to minimize emissions of compounds that deplete the ozone layer and to monitor it are essential. What Is Montreal Protocol?The goal of the Montreal Protocol is to preserve the ozone layer by gradually banning the manufacture and use of chemicals that damage it. The protocol was signed in Montreal, Canada, on September 16, 1987, and has been widely regarded as one of the most successful international environmental agreements ever created. The Montreal Protocol's main objective is to address the usage of ozone-depleting compounds such carbon tetrachloride, halons, and chlorofluorocarbons (CFCs). These substances were commonly used in refrigeration and air conditioning systems, aerosol sprays, and foam insulation, among other applications. Once released into the atmosphere, they can break down ozone molecules in the stratosphere, causing a depletion of the ozone layer. The protocol established a schedule for phasing out the production and consumption of these substances, with developed countries beginning to phase out CFCs by 1996 and developing countries starting in 2010. The protocol also established a fund to help developing countries transition to alternative technologies and substances that do not harm the ozone layer. The Montreal Protocol was a significant achievement in international cooperation, as it required cooperation among many countries with varying economic and political circumstances. It is frequently cited as an illustration of how international collaboration may assist in resolving major environmental issues. The substantial decrease in ozone-depleting compounds in the atmosphere is evidence of the Montreal Protocol's success. According to the United Nations Environment Programme (UNEP), the protocol has led to the reduction of over 98% of ozone-depleting substances in the atmosphere. The protocol has also helped to mitigate climate change, as many of the substances it regulates are also potent greenhouse gases. The protocol has been amended several times since its inception, with each amendment strengthening its provisions and expanding its scope. For example, the Kigali Amendment, which was added to the protocol in 2016, addresses the use of hydrofluorocarbons (HFCs), which are potent greenhouse gases that do not deplete the ozone layer. Despite its success, the Montreal Protocol is an ongoing effort that requires continued cooperation and action. The fund established by the protocol needs to be replenished regularly to support the transition to alternative technologies, and monitoring of the ozone layer needs to continue to ensure that it continues to recover. Montreal Protocol is a significant achievement in international cooperation and a model for addressing global environmental challenges. Its success demonstrates the potential for international cooperation to address global environmental issues and serves as a reminder of the importance of continued action to protect the Earth's ozone layer. What Is An Ozone Hole?The ozone hole is a region in the stratosphere of the planet where the amount of ozone is extremely low. The hole was first discovered in the 1980s and has been a cause for concern ever since, as it poses a significant threat to the Earth's environment and human health. Ozone is a gas that is naturally present in the Earth's atmosphere and plays a crucial role in protecting the planet from the harmful effects of the sun's ultraviolet (UV) rays. 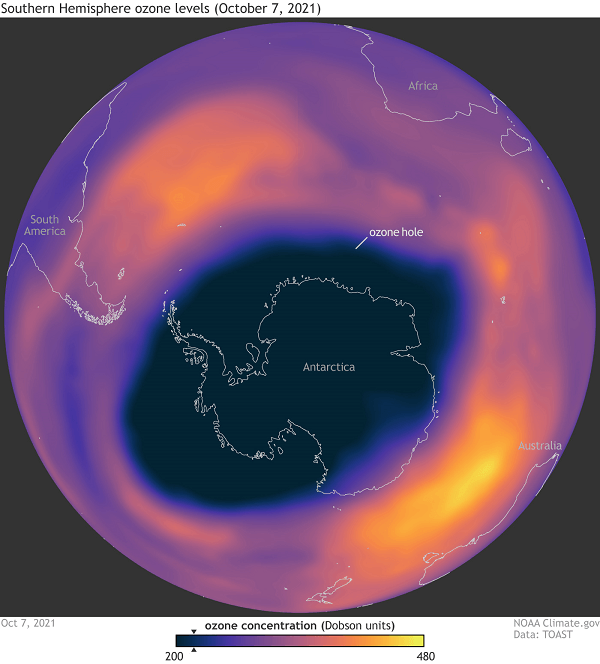
A portion of the stratosphere's ozone layer is where there is a concentrated amount of ozone, which absorbs UV radiation from the sun before it reaches the Earth's surface. Some compounds in the atmosphere, such as chlorofluorocarbons (CFCs), halons, and other ozone-depleting substances, contribute to the ozone layer's thinning (ODSs). These substances were frequently employed in foam insulation, aerosol sprays, air conditioning, and other industrial operations. Once released into the atmosphere, these chemicals rise up to the stratosphere and break down ozone molecules through a chemical reaction, leading to the formation of an ozone hole. The destruction of ecosystems and increased risk of skin cancer, cataracts, and other illnesses in both humans and animals are two detrimental effects of the ozone layer's depletion. The ozone hole was first discovered over Antarctica in the late 1980s by a team of scientists from the British Antarctic Survey. Since then, the hole has been monitored regularly using satellites, balloons, and ground-based instruments, and its size and severity have fluctuated over time. The size of the ozone hole varies depending on the time of year, with the largest hole typically occurring during the Antarctic spring (September to November) when the sun's rays begin to return to the region after the long polar night. During this time, the temperature in the stratosphere drops, which creates ideal conditions for the formation of the ozone hole. After widespread concern over the ozone hole, the Montreal Protocol, which aimed to gradually phase out the manufacturing and consumption of ozone-depleting compounds, was signed in 1987. The protocol has been successful in lowering the production and usage of ODSs and was largely considered as a notable achievement in international collaboration. As a result of the Montreal Protocol, the production and consumption of most ODSs have been phased out in developed countries, and developing countries are also making progress towards phasing them out. The recovery process is slow because ozone-depleting substances can remain in the atmosphere for decades, and the natural processes that create ozone also take time. The ozone hole is a significant environmental issue that requires ongoing attention and action. The ozone hole will remain a problem for years to come even if the Montreal Protocol has been successful in decreasing the production and use of ozone-depleting compounds. Continued monitoring and research are necessary to understand the causes and consequences of the ozone hole and to develop effective solutions to protect the Earth's environment and human health. Ozone Layer RecoveryA vital part of the Earth's atmosphere, the ozone layer shields us from the sun's harmful UV radiation. In the late 20th century, scientists discovered that human-made chemicals known as chlorofluorocarbons (CFCs) and halons were damaging the ozone layer, creating an "ozone hole" above Antarctica. Fortunately, global efforts to curb the use of these chemicals have helped to initiate the recovery of the ozone layer. 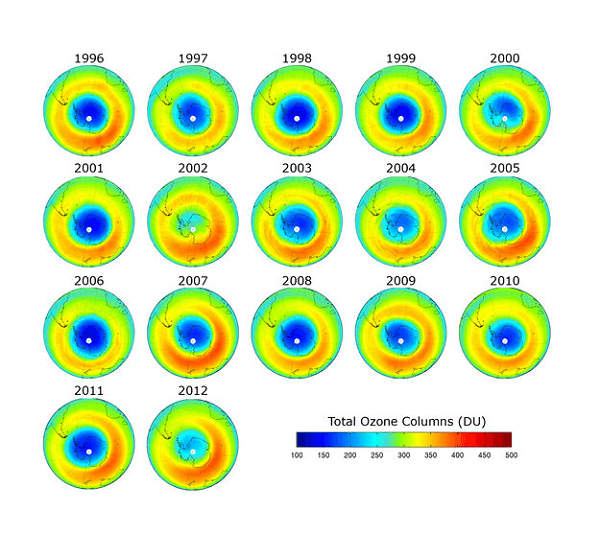
The first major international effort to protect the ozone layer was the Montreal Protocol, signed in 1987. The treaty set out to phase out the production of ozone-depleting substances (ODSs) including CFCs, halons, and other ozone-depleting chemicals. The protocol has been successful in reducing the production and consumption of ODSs, with a phase-out timetable that is currently being followed by over 190 countries. Over the past several decades, scientists have been monitoring the ozone layer to determine the extent of the damage caused by ODSs and to track the recovery process. The most important indicator of ozone depletion and recovery is the size and depth of the ozone hole over Antarctica. Recent studies have shown that the size of the Antarctic ozone hole has steadily decreased since the late 1990s, indicating that the ozone layer is recovering. In 2019, the ozone hole reached its smallest size on record since its discovery, with a peak area of 9.3 million square kilometers. This recovery can be attributed to the success of the Montreal Protocol, which has helped to reduce the production and consumption of ODSs worldwide. ODSs can persist in the atmosphere for decades, so the impact of the Montreal Protocol has taken time to be fully realized. Nevertheless, the long-term effects of the treaty are encouraging. In addition to the Montreal Protocol, other factors have contributed to the recovery of the ozone layer. Changes in atmospheric circulation, which are partly caused by climate change, have helped to stabilize the ozone layer over Antarctica. Reductions in emissions of air pollutants such as nitrogen oxide and methane have also contributed to ozone recovery, as these pollutants can destroy ozone in the lower atmosphere. Despite the progress that has been made, there are still challenges to be faced in the recovery of the ozone layer. One of the main challenges is the ongoing presence of ODSs in the atmosphere, which can continue to damage the ozone layer for many years to come. It is therefore important to continue monitoring the atmosphere to ensure that the Montreal Protocol and other measures are effective in protecting the ozone layer. Another challenge is the potential for new chemicals to emerge that may be harmful to the ozone layer. For instance, a class of compounds known as hydrofluorocarbons (HFCs) has been utilised in several applications to replace CFCs. While HFCs do not directly deplete the ozone layer, they are potent greenhouse gases that contribute to climate change, which in turn can affect the recovery of the ozone layer. To address these challenges, the international community must continue to work together to ensure that the Montreal Protocol and other agreements are effective in protecting the ozone layer. This may involve developing new technologies that can replace ODSs in industrial and consumer applications, as well as continued monitoring and research to understand the complex interactions between the ozone layer and other components of the Earth's atmosphere. The recovery of the ozone layer is a significant environmental achievement that demonstrates the power of international cooperation to address global environmental challenges. While challenges remain, the progress made so far is encouraging and serves as a reminder of the importance of continued vigilance in protecting the Earth's environment. Alternative RefrigerantsChemicals called refrigerants are used in refrigeration and air conditioning systems to move heat from one location to another. Traditionally, the most commonly used refrigerants have been hydrochlorofluorocarbons (HCFCs) and chlorofluorocarbons (CFCs), which have been linked to ozone depletion and climate change. The search for environmentally friendly substitute refrigerants has intensified in recent years. Hydrofluorocarbons (HFCs), a type of substitute refrigerant, don't contain chlorine and don't deplete the ozone layer as a result. HFCs, however, are strong greenhouse gases that fuel climate change. The Kigali Amendment to the Montreal Protocol, which aims to gradually phase out HFCs in favour of low global warming potential (GWP) refrigerants, was approved in 2016. One such low-GWP refrigerant is hydrofluoroolefins (HFOs), which are similar in structure to HFCs but have much lower GWP values. HFOs are currently used in some refrigeration and air conditioning systems, but their production is still relatively small compared to HFCs. Another alternative refrigerant is carbon dioxide (CO2), which is a naturally occurring gas that is non-toxic and non-flammable. CO2 refrigeration systems have been used in some commercial and industrial applications, but they are not yet widely used in residential applications due to their higher upfront cost and the need for specialized equipment. Another substitute refrigerant that is frequently utilised in commercial refrigeration systems is ammonia (NH3). Ammonia is a natural refrigerant that has a low GWP and is non-ozone depleting, but it is toxic and flammable, which can make it unsuitable for some applications. Finally, there are also natural refrigerants such as propane, butane, and isobutane that have been used in smaller refrigeration systems such as household refrigerators and freezers. These natural refrigerants are non-ozone depleting and have low GWP values, but they are flammable and require specialized equipment and safety precautions. While alternative refrigerants offer promising solutions to the environmental impacts of traditional refrigerants, there are still challenges to their adoption. One major challenge is the need for retrofitting or replacing existing refrigeration and air conditioning systems, which can be costly and time-consuming. In addition, there are safety concerns related to some alternative refrigerants such as ammonia and flammable natural refrigerants. 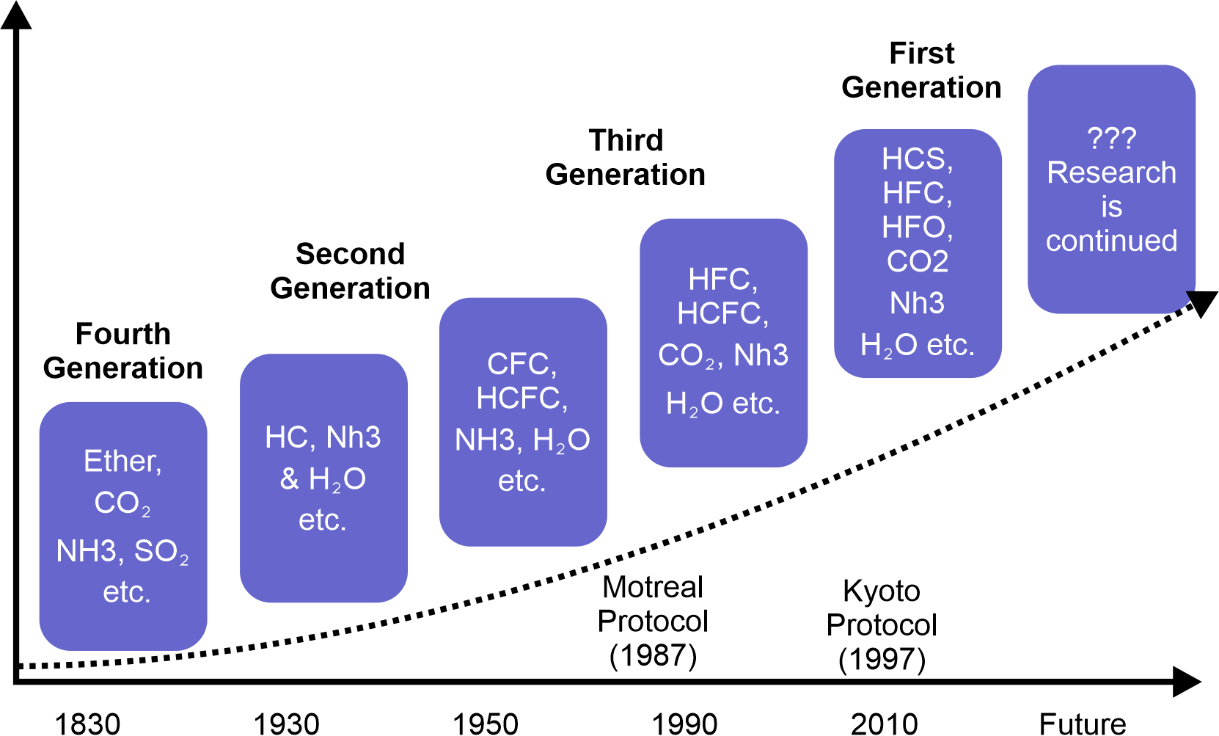
Overall, the search for alternative refrigerants is an important part of global efforts to address climate change and protect the ozone layer. While there is no one-size-fits-all solution, a variety of alternative refrigerants are available that can be tailored to specific applications and requirements. It is probable that new and even more environmentally friendly refrigerants will be created as technology advances. Relation Between Ozone and Climate Change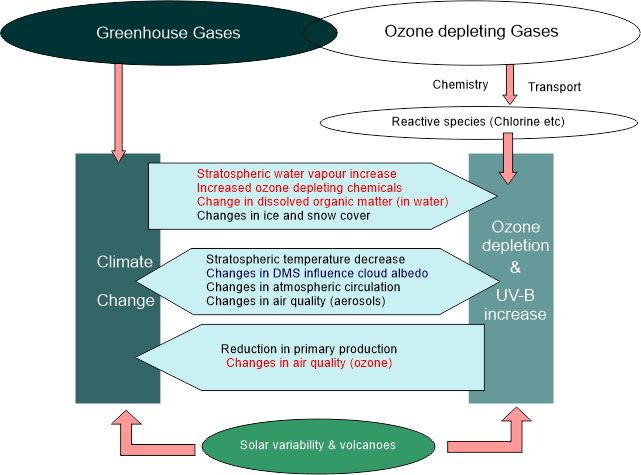
The ozone layer and climate change are two distinct environmental issues that are often discussed separately. However, there is a relationship between the two, as both are influenced by human activities and can have significant impacts on the Earth's atmosphere and ecosystems. The ozone layer is a protective layer of gas in the stratosphere of the Earth that absorbs most of the sun's ultraviolet (UV) light, which can harm both people and animals by causing skin cancer, cataracts, and other health issues. Ozone depletion occurs when chemicals such as chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are released into the atmosphere, breaking down ozone molecules and reducing the layer's thickness and effectiveness. The long-term alteration of the Earth's climate brought on by human activities like the combustion of fossil fuels, deforestation, and industrial agriculture is referred to as climate change, on the other hand. Increasing atmospheric levels of greenhouse gases including carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) have had a negative impact on climate, causing global temperatures to rise, as well as altered weather patterns and sea level rise. The ozone layer and climate change have a complicated and intertwined interaction. One way in which they are connected is through the role of ozone-depleting substances (ODS) in contributing to climate change. ODS such as CFCs and HCFCs not only harm the ozone layer but also have potent global warming effects as greenhouse gases. Furthermore, changes in temperature and weather patterns due to climate change can affect the distribution and transport of ozone in the atmosphere. For example, warming temperatures in the lower atmosphere can alter wind patterns and impact the movement of ozone from the tropics to higher latitudes. Changes in atmospheric circulation and precipitation can also affect the chemistry of the stratosphere, which can impact the ozone layer. In addition, changes in UV radiation due to ozone depletion can have indirect effects on climate. UV radiation affects the growth and photosynthesis of plants and phytoplankton, which are important carbon sinks that absorb and store CO2 from the atmosphere. As a result, variations in UV radiation levels may have an effect on the global carbon cycle and fuel climate change. Efforts to address both ozone depletion and climate change have been intertwined, with the Montreal Protocol playing a key role in phasing out ODS and reducing both ozone depletion and global warming. The 2016 Montreal Protocol Kigali Amendment aims to gradually phase out the use of hydrofluorocarbons (HFCs), powerful greenhouse gases that are frequently employed as refrigerants, and replace them with low-global-warming-potential substitutes. ConclusionIn conclusion, the ozone layer and climate change are two interconnected environmental issues that are influenced by human activities and have significant impacts on the Earth's atmosphere and ecosystems. Addressing one problem can have benefits for the other, and efforts to reduce ozone-depleting substances and greenhouse gases are critical for protecting both the ozone layer and the climate.
Next TopicPast Tense Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










