Phosphoric AcidPhosphoric acid is also called orthophosphoric acid. It is a weak, inorganic acid that is obtained from the mineral phosphorus. In raw form, it can be obtained from phosphate rocks, whereas, in pure, it is produced in the industries from white phosphorus. In pure form, it exists in the solid crystalline state. In the liquid state, it is colorless, odorless and non-volatile and is less concentrated. Further, it can be found in various foods and beverages such as soft drinks, dairy products, cereal bars, flavored waters, and processed meats. Chemical Structure of Phosphoric AcidThe chemical or molecular formula of phosphoric acid is H3PO4. So, it is made of one phosphorus atom, four oxygen atoms and three atoms of hydrogen. 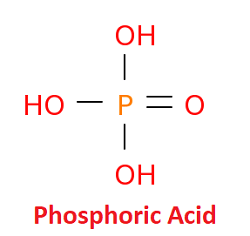
It is a phosphorus oxoacid that is made of one oxo and three hydroxy groups bonded to phosphorus atom by covalent bonds. The phosphorus at center is connected to the oxygen atom by a double bond, whereas, it is connected to three hydroxyl groups (-OH) through single bonds. So, it can donate up to three H+ ions. The three hydrogen atoms are acidic in nature and can separate themselves from the molecule as H+ ions called protons. When it happens or all hydrogen atoms leave the molecule, the orthophosphate ion (PO43-) is formed. This ion is known as phosphate. If one proton is removed, it gives dihydrogen phosphate ion H2PO- 4, similarly, if two protons are removed, it forms hydrogen phosphate ion HPO42-. Phosphoric acid is safe for children and it does not contain genetically modified organisms (GMOs). It has been used in food since 1885. It was used first in the preparation of formulation of beverage that later known as Coca-Cola. It was discovered independently by two Swedish chemists as a part of bone ash in 1770. After four years, Scheele found that this acid can be made by adding nitric acid to phosphorus. Physical Properties of Phosphoric acid
Chemical Properties of Phosphoric acid
Preparation of Phosphoric AcidThere are two different processes or methods for the preparation of phosphoric acid as described below; 1. Wet ProcessIn this method, it is prepared from a crystal rock fluorapatite, which is found naturally and contains the phosphate mineral. The fluorapatite is reacted with concentrated sulphuric acid H2SO4 and water H2O to form phosphoric acid and calcium sulfate or gypsum with some amount of insoluble impurities. The unwanted chemical compounds and impurities are separated through filtration and evaporation. Later the acid is concentrated to 56% to 70% P2O5 through vacuum distillation. The reaction takes place as shown below; Ca5 (PO4)3Cl + 5H2SO4 + 10H2O → 3H3PO4 + 5CaSO4.2H2O + HCl The acid formed in this method is an impure acid. It can be used in the manufacturing of fertilizers without purifying it further. 2. Thermal ProcessIt is another method to produce phosphoric acid. In this method, phosphorus is heated or burnt at high temperature in the presence of atmospheric oxygen. It leads to the formation of phosphorus pentoxide, which is condensed to form a white powdery substance. Then it is hydrated by a separate process to get phosphoric acid. Furthermore, in some cases, steam is also supplied to the burner to form a condensed form of polyphosphoric acid. This acid is then made to pass through a hydration tower where the gaseous phosphorus oxide is absorbed and phosphoric acid is obtained. Phosphoric Acid SaltsThey are called phosphates, some of the important phosphates are described below; 1. Ammonium phosphatesDiammonium hydrogen and monoammonium dihydrogen phosphates are commonly used as fertilizers. These phosphates are obtained by mixing a specified quantity of phosphoric acid with anhydrous ammonia in a revolving drum. 2. Calcium PhosphatesThey are widely used as fertilizers. They are produced by reacting sulfuric acid with phosphate rock. The reaction that takes place is shown below: Ca3(PO4)2 + H2SO4 →Ca(H2PO4)2 + 2CaSO4 This phosphate is superphosphate with 20% P2O5. When phosphate rock is reacted with phosphoric acid other than sulfuric acid, a more concentrated form of calcium dihydrogen phosphate is produced that is with 55% P2O5. The reaction is given below: Ca3(PO4)2 + 4H3PO4 → 3Ca(H2PO2)2 Triple superphosphate is produced in the above reaction. This phosphate level is obtained as the product is not diluted with calcium sulfate in this case. 3. Sodium PhosphatesThis type of phosphates is formed when phosphoric acid is reacted with a concentrated solution of sodium hydroxide. The phosphates are formed in the form of solid crystals. Some examples are Disodium hydrogen phosphate, Trisodium phosphate, Monosodium dihydrogen phosphate, etc. Phosphoric Acid UsesPhosphoric acid offers lots of uses. It is used in several industries, agriculture and products that we use. Here are some of its major uses: Removal of Rust It is used for removing rust from metals like iron, steel, and more. When it is applied to the rust, it reacts with the rust and changes the reddish-brown iron into a black-colored ferric phosphate, which can be removed easily. Food and beverage industry It is widely used as a food additive. It regulates the acidic character of foods like jams, cheese, processed meats, and more. Further, in the beverage industry, it is used as an acidulant, which gives specific flavors to foods. It is also used to check the growth of fungi and bacteria. Personal care It is widely used in the preparation of a large variety of personal care products such as cleaning products, bath products, hair care products, makeup items, etc. further, it is also used to control the pH of these products. In Agriculture: It is also used in the production of fertilizers. Further, it is also used as a flavoring agent in animal and poultry foods. Pharmaceutical: In the pharma industry, it is used as an intermediate. It is also used in dentistry to clean teeth. It is also found in teeth whiteners or mouth washes. Further, it is also an ingredient in anti-nausea medicines.
Next TopicBoric Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










