Picric AcidPicric acid is an organic compound with the chemical formula C6H3N3O7 or C6H2(NO2)3OH. It is a type of phenol. It is also called 2,4.6-Trinitrophenol (chemical name). It has a bitter taste, so it is named picric acid. The word picric is derived from the Greek word (pikros), which means "bitter". It is known to be highly acidic among all the phenols. So, if it is hit or heated, it can explode. Its salts that contain heavy metals can also explode if heated or hit continuously. Structure of Picric acidIt has a phenyl group to which three electron-withdrawing groups (NO2) are attached at the ortho and para positions of phenol. 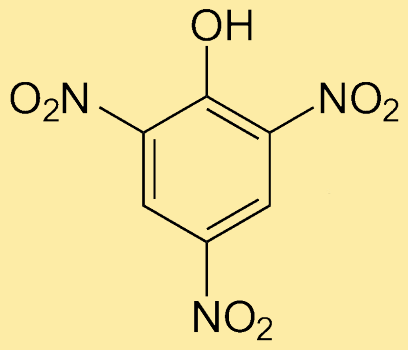
History of Picric AcidPicric acid was created by British scientist Peter Woulfe in 1771. He created it by reacting indigo with nitric acid. In the mid of 19 century, initially, it was used as a yellow dye for silk. Later, it was highly valued as an explosive called melinite and was used for the first time to aid the bursting of shells by the French in 1886. During the Russo-Japanese, it was the widely used explosive by the military. In modern times, shells use ammonium picrate (a salt of picric acid) owing to its ability to tolerate the strong shock of penetration before detonating. Further, it has both antibacterial and astringent properties. Properties of Picric Acid
Preparation of Picric AcidIt is mainly prepared from phenol. The phenol is reacted with concentrated sulphuric acid and concentrated nitric acid. The nitro groups of nitric acid get transferred to the ortho and para positions of phenol. These positions provide stability to the newly formed picric acid. The reaction is shown below: 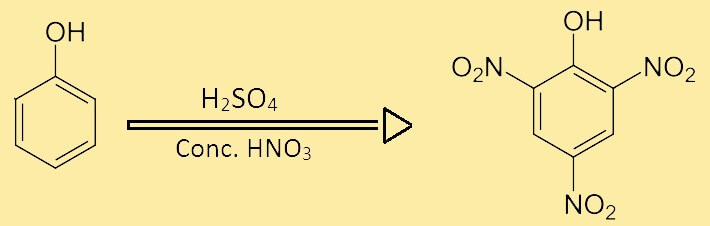
The above process is completed in two steps. In this first step, the formation of phenol sulphuric acid takes place which in the second step reacts with conc. Nitric acid to form 2,4,6- trinitrophenol, which is called picric acid. Uses of Picric AcidPicric acid offers lots of uses, some of them are as follows:
Health HazardsPicric acid is toxic in nature. If it is happened to swallow by someone or absorbed through the skin, it may cause health issues. For example, inhalation of its dust may cause lung damage and long-term exposure may affect the liver or kidney. So, while using picric acid one should wear protective clothing, avoid skin contact and use gloves, face masks and goggles to avoid inhalation of dust. How to check Picric Acid ContainersThe picric acid containers should be checked on a quarterly basis. Any trained person can do this. How one can self-inspect is described below:
Storage Instructions
Next TopicHow to Cure Acidity Permanently
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










