Pressure DefinitionThe concept of pressure is a fundamental aspect of physics that is relevant in our daily lives. It refers to the force applied on a given unit area and governs common devices like hydraulic systems and tires' behavior and fluids and gases' behavior. Comprehending pressure is vital across various fields, including meteorology, engineering, and physics. Hence, studying this concept is crucial to understand its implications thoroughly. 
PressurePressure is the measured force exerted per unit area, denoting the force applied per unit area to a surface. Various units such as Pascals (Pa), pounds per square inch (psi), and atmospheres (atm) are used to express pressure. Pressure is based on the concept that when a force is exerted on a surface, it generates a perpendicular force distributed throughout the surface. The pressure is calculated by dividing the force by the surface area, as the force is spread out across the whole surface. Therefore, pressure decreases as the surface area increases and increases as the surface area decreases. Various instruments are available to measure pressure, including pressure gauges, manometers, and barometers. These instruments determine the force acting on a particular area and transform it into a pressure reading. In science and engineering, pressure holds great significance with several important applications. One such example is its role in "fluid mechanics", which is vital in governing fluid flow. Studying "atmospheric pressure changes" is also crucial for predicting weather patterns. Furthermore, pressure is a critical factor in the operation and design of numerous industrial processes and equipment, including compressors, boilers, pipelines, etc. So, pressure is a fundamental physical quantity essential for understanding many natural and technological systems. 
Types of pressureThere are several types of pressure, including:
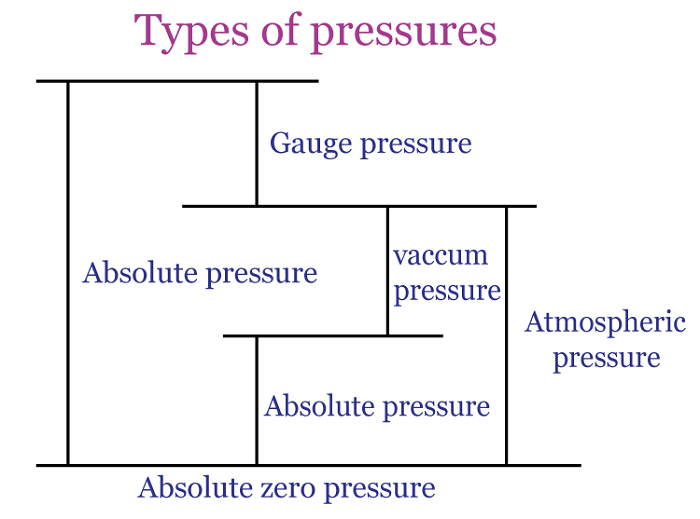
Atmospheric pressureAtmospheric pressure is the weight of air above any point on the Earth's surface exerting force. The air surrounding the Earth generates this pressure, which is calculated based on the combined weight of the air molecules. Air molecules at higher altitudes have less pressure as they are subjected to fewer molecules pushing down from above. In contrast, lower altitudes molecules are subject to greater pressure from the piled-up molecules and are more densely packed. Represented by the symbol "atm," the familiar atmosphere is a pressure unit defined as 101325 Pa (1.01325 bar). For example: When a straw is placed in water, and the air is removed from the opposite end, it decreases the pressure within it. Nevertheless, the pressure exerted by the atmosphere on the water's surface results in water being pushed out of the straw. Another example: A rubber sucker adheres to a flat surface, resisting removal when pulled. This occurs because most of the air between the sucker and the surface escapes, resulting in minimal air inside. The atmospheric pressure from outside holds the sucker in place, causing it to remain stuck. To detach the sucker, a force greater than atmospheric pressure must be applied. 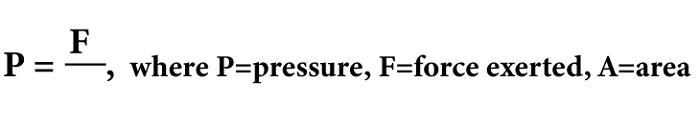
Hydrostatic pressureHydrostatic pressure is the pressure exerted by a fluid at rest on an object or surface in contact with it. This pressure is caused by the weight of the liquid above the object or surface, increasing with the fluid's depth and density. The deeper the object is submerged, the higher the hydrostatic pressure. This phenomenon is important in many fields, including fluid mechanics, engineering, and geology. For example: A water tower is a raised tank filled with water through pumps. The hydrostatic pressure from this setup allows the water to be directed into nearby homes without extra pumps. The tank is typically quite large and can hold millions of liters of water, so the water level drops slowly. It helps to maintain a consistent water pressure until the tank needs to be refilled once the water level reaches a specific threshold. 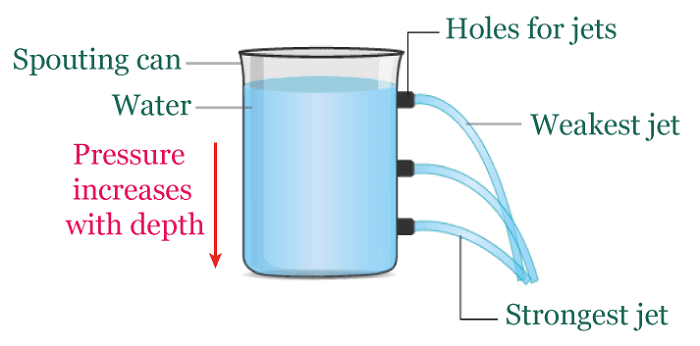
Absolute pressureAbsolute pressure is the total pressure measured against a vacuum reference point, and it results from adding air pressure to any pressure caused by a liquid or gas. Absolute pressure (Pa) is commonly measured using psi (pounds per square inch) or pascals. The calculation for absolute pressure is as follows. Absolute pressure = Gauge pressure + Atmospheric pressure Absolute pressure is usually measured in units of pounds per square inch (psi) or pascals (Pa). For example: Imagine a balloon filled with air at sea level. The pressure inside the balloon is not just the pressure of the air inside it but also the pressure of the atmosphere surrounding it. If we measured the pressure inside the balloon with a gauge, we would get a reading of the gauge pressure. This gauge pressure only accounts for the pressure of the air inside the balloon, not the pressure of the atmosphere around it. We must add the atmospheric pressure to the gauge pressure to know the absolute pressure inside the balloon. For example, if the gauge pressure is 20 kPa, and the atmospheric pressure is 101.325 kPa, then the absolute pressure inside the balloon would be 121.325 kPa. Gauge pressureGauge pressure is the measure of pressure that takes into account atmospheric pressure. It is calculated by subtracting the local atmospheric pressure from the absolute pressure. The gauge pressure can be either positive or negative, depending on whether it is greater or less than the atmospheric pressure. To calculate the gauge pressure, one can use the following formula: Gauge pressure = Absolute pressure - Atmospheric pressure As an illustration, a car tire is inflated to 30 PSI (pounds per square inch). The tire pressure gauge reads 30 PSI above the atmospheric pressure. The atmospheric pressure is usually around 14.7 PSI, so the gauge pressure in the tire is 30 - 14.7 = 15.3 PSI. Gauge pressure is used in many industries, such as HVAC, pneumatic and hydraulic systems, to measure and control pressure. It is important to understand the concept of gauge pressure as it can affect the performance and safety of these systems. 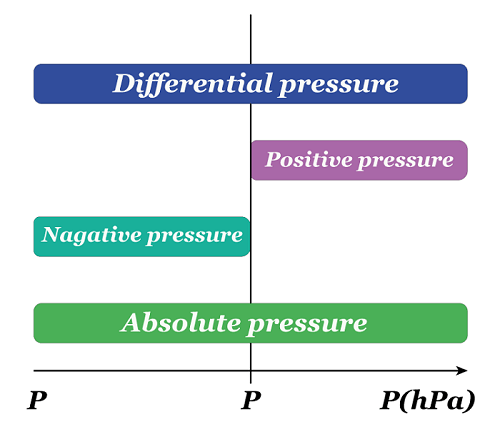
Differential pressureThe disparity in pressure between two locations in a fluid system is known as differential pressure. This pressure difference is an important concept in many engineering and scientific applications, where it is used to measure flow rates, fluid levels, and other variables. It is quantified in pressure units, such as pounds per square inch (psi) or pascals (Pa). The calculation for "differential pressure" is as follows. Differential pressure = Pressure 1 - Pressure 2 To understand differential pressure, consider the example of a flowmeter used to measure the flow rate of a liquid. The flowmeter is connected to two points in the fluid system: the upstream point, where the liquid enters the flowmeter, and the downstream point, where it exits. As the liquid flows through the flowmeter, it encounters resistance due to its internal design. This resistance causes a pressure drop between the upstream and downstream points, creating a pressure difference. The flowmeter measures this pressure difference and uses it to calculate the flow rate of the liquid. As the pressure difference increases, the rate of flow also increases. Another example: If the pressure at the upstream point is 50 psi and the pressure at the downstream point is 45 psi, the differential pressure is five psi. This pressure difference is used to calculate the flow rate of the liquid, which can be used to monitor and control the flow of the liquid through the system. 
Osmotic pressureOsmotic pressure, or osmosis, is the lowest amount necessary to stop solvent molecules from slipping through the semipermeable membrane. The concentration of solute particles in the solution is a determining factor in this property, known as a colligative property. The formula used to determine osmotic pressure is: π = iCRT For example: The upright posture of plants is maintained through the use of osmotic pressure. Cells within the plant, containing various salts, absorb water and expand when adequate water is available. This expansion causes an increase in pressure against the cell walls, resulting in the plant standing tall. Conversely, a lack of water leads to hypertonic cells, causing the plant to wilt and lose its firmness. The measurement of osmotic pressure can also be used to determine the molecular weight of substances. Vapour pressureThe inclination of a substance to transform into a gaseous form is known as vapour pressure. Typically, vapour pressure denotes the pressure generated by the liquid's vapour, which is in thermodynamic equilibrium with the condensed phases in a closed system. The vapour pressure of liquid augments with the escalation of its temperature. There are multiple ways to determine the vapour pressure of a liquid. The most straightforward technique involves utilizing a manometer within a sealed container or flask. For example: A tea kettle best illustrates the phenomenon of vapour pressure. As the water inside the kettle reaches boiling point, the water vapours increase, which applies pressure on the kettle lid, resulting in an upward movement. 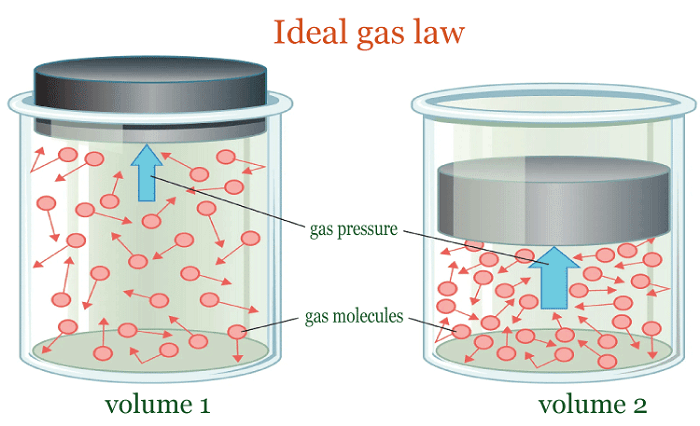
Vacuum pressureVacuum pressure exists in a space where the gas or air pressure is lower than the surrounding atmospheric pressure. It is typically measured in pressure units, such as pascals (Pa) or inches of mercury (inHg). It is often used in vacuum pumps, air conditioning systems, and scientific experiments. The pressure is very low in a vacuum, and there are few or no gas molecules. This creates a low-pressure environment that can be used for various purposes, including removing air from containers, sterilizing equipment, and preserving food. The pressure can be used in vacuum pick and place applications, observed across various sectors, including automotive, food and beverage, manufacturing, pharmaceutical, chemical, and nautical industries.
Next TopicRadiation Definition
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










