SolutionA solution is a homogenous mixture of two or more components in which particle size is generally less than 1 nm. Solutions are part of our life as there are lots of things that we use every day are solutions such as cold drinks, deodorant, milk, tea, soups, creams, and more. 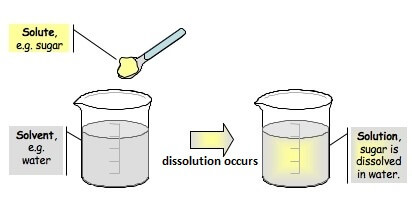
A solution does not need to be in a liquid state always. They can occur in any state of the matter such as liquid, solid or gas. For example, steel is also a solution of iron and copper. Similarly, air is a solution of oxygen, nitrogen and other gases and soft drink is a solution in which gas is dissolved in liquid. So, a solution can exist in any three states of matter. Components of a SolutionThe substances that form a solution by mixing with each other are called components of a solution. Generally, a solution is made of two components that include solvent and solute. i) Solvent: It is the component of a solution that is present in the largest amount and dissolves the other component that is present in small quantity in it. The solvent tends to dissolve solute by pulling its molecules or particles apart and thus allows solute particles to diffuse throughout the solvent until the solute is uniformly distributed or dissolved in the solution. So, we can say that when solutes get dissolved in the solvent a solution is formed. Besides this, a solution always has one solvent as the largest component of the solution. For example, in seawater, water is the solvent and all other substances dissolved in it like metals, salts and gases are solutes. ii) Solute: It is the component of a solution that is present in small quantity and dissolves in the solvent. There can be one or more solutes dissolved in the solvent. For example, in saltwater, salt is solute and water is solvent, similarly, in carbonated drinks the dissolved carbon dioxide gas is the solute. A solute can be of two types based on its solubility as follows; Soluble solute: A solute is called soluble if its 0.1 gm or more can be easily dissolved in 100 ml of the solvent. Sparingly soluble: The solute is said to be sparingly soluble if less than 0.1 gm of it dissolves in 100 ml of the solvent. So, we can say that a solution is a homogenous mixture of solute and solvent molecules. The state of the solvent generally determines the final state of the homogenous solution. The state of the solute does not affect the solution as long as they are soluble in the solvent. Furthermore, water is considered a universal solvent as it can dissolve or we can dissolve most of the substances in it. However, there are also many substances that do not dissolve in water, so there are various other substances that are also used as solvents such as alcohol, ether, benzene, carbon tetrachloride, carbon disulphide. A solution in which water is present in large amount and acts as solvent by dissolving solutes is called an aqueous solution. For example, a solution of sugar and water is an aqueous solution. Similarly, a solution that has any substance as solvent other than water is called a non-aqueous solution. For example, iodine dissolved in alcohol is a non-aqueous solution. Furthermore, a solution that is made of one solvent and one solute is called a binary solution, e.g. solution of salt and water. Similarly, if there are two solvents and one solute, or one solvent and two solutes, it is called a tertiary solution, e.g. solution of sugar, salt and water. Characteristics of Solutions
Although a solution is a homogenous mixture of two or more components, not all mixtures can be considered a solution. Only those mixtures that fulfil the following conditions can be considered solutions.
How is a Solution formed?A force of attraction exists between constituting particles or molecules of a substance. This intermolecular force of attraction also exists in solutions and is responsible for the formation of solutions. In solutions, the force of attraction, which exists between solvent molecules is called solvent-solvent interactions and that exists between solute molecules is called solute-solute interactions, and interactions between solute and solvent are called solvent-solute interaction. When we mix two substances (solvent and solute) the solvent and solute particles or molecules interact with each other. The interaction is of three type solvent-solvent, solute-solute and solvent-solute interaction. A solution is formed when these interactions are of the same strength and type. So, we can say that the formation of a solution depends on these interactions. Let us see how does a solute dissolve in a solvent? Let us understand it with an example of salt water. When salt is added into plain water, the salt crystals start interacting with water molecules. A salt crystal consists of sodium ion (Na+) and chloride ion (Cl-), which form a cubic lattice. The ions present at the corners or edges of this lattice structure are loosely held. Now, salt crystals interact with water molecules which are polar in nature as in a water molecule (H2O) hydrogen atom carries a partial positive charge and oxygen atom carries a partial negative charge, as oxygen is more electronegative than hydrogen. The water molecules collide with salt crystals and the sodium and chlorine ions break free. The sodium ions are positively charged and chlorine ions are negatively charged and water molecules are also polar so water molecules get attracted or move towards salt ions and form a cluster around them. The oxygen atom which has a partial negative charge move towards positively charged sodium ion and positively charged hydrogen atoms of water molecules tend to gather around negatively charged free chloride ion. Thus, water molecules cluster around ions to stabilize them through ion-dipole interaction. This process in which water is absorbed or a substance combines with water is called hydration. The hydration or dissolution of ion crystals continues until all free crystals combine with water molecules and thus solute particles get dissolved in a solvent to form a solution. This process is known as dissolution. However, besides dissolution, another process also takes place during the formation of a solution such as crystallization. For example, even after the dissolution of crystals or ions, sometimes the free ions of sodium and chlorine collide with salt crystals and due to the strong interaction between them, the ions again become part of the crystal and thus get separated from the solvent. This process is opposite to that of dissolution and thus is called crystallization. So, even after the formation of the solution, dissolution and crystallization keep occurring simultaneously and create a dynamic equilibrium in the solution. 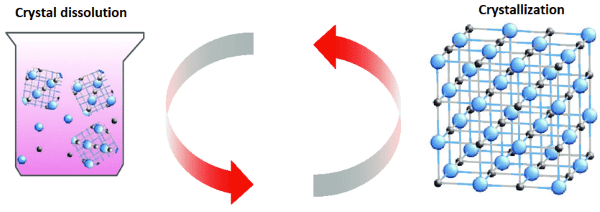
However, in the beginning, the rate of crystallization is more than the rate of crystallization. It means, in the beginning, more solute particles are mixing with the solvent and less solute particles are separating out. But, if we add more salt (solute) to the solution, the rate of dissolution tends to decrease and the rate of crystallization increases and a point is reached when the rate of dissolution becomes equal to the rate of crystallization. However, from outside, there is no visible change if you look at the container. We can say that no more salt is dissolving in the solution. However, at the molecular level solute particles are entering and leaving the solution at equal rates. So, at this stage, the solution is in dynamic equilibrium with the undissolved solute. A solution in which we cannot dissolve more solute at a given temperature and pressure is called a saturated solution. So, in a saturated solution, we have reached the maximum amount of solute that can be dissolved in a solution at a given temperature and pressure. This maximum amount of solute that can be dissolved in a solution is called the solubility of the solution. At this point, the rate of dissolution and rate of crystallization becomes equal and no more solute can be added to the solution.
Next TopicTypes of Solutions
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










