Sulphuric Acid
Sulphuric acid is a mineral acid. It is an oily liquid with no odor and no color. It is also called Oil of Vitriol. It offers lots of uses owing to this it is also called as the 'Kind of Chemicals'. It exists in both combined and free states.
Sulphuric acid chemical formula
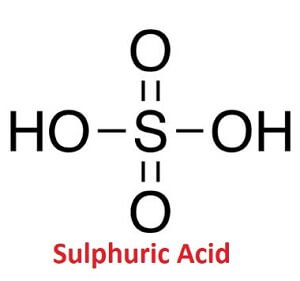
The chemical formula of sulphuric acid is H2SO4. It is made of one Sulphur atom that is bonded to two oxygen atoms by double bonds and bonded to two hydroxyl groups (OH) by single bonds.
Physical properties of sulphuric acid
- It is colorless and odorless.
- It is an oily and thick liquid.
- Its specific gravity is 1.84 at 298 K.
- The boiling point of the acid is 611 K.
- The high boiling point is because of hydrogen bonding.
- It is a strong acid, which makes it react with water vigorously. So, water should not be added to sulphuric acid, however, acid can be added to water slowly with proper stirring.
Chemical Properties of Sulphuric Acid
- It is a strong dibasic acid; it ionizes in an aqueous solution.
- It has a high corrosive nature, is highly reactive and is soluble in water.
- Its oxidizing power is high as it is a strong oxidizing agent. It can oxidize both metals and non-metals. However, it is reduced to Sulphur dioxide.
- Its volatility is low; owing to this reason, it can be used to prepare more volatile acids from their matching salts.
- It is also a powerful dehydrating agent. So it can dry many wet gases that do not undergo reaction with the acid.
- It can remove water from natural mixes like starches.
Some Common Reactions of Sulphuric Acid
Copper is oxidized to copper sulfate when reacts with hot concentrated sulphuric acid, as shown below:
Cu + 2H2SO4 → CuSO4 + SO2 + H2O
Concentrated sulphuric acid when reacts with sodium chloride forms hydrogen chloride. Similarly, it produces hydrogen fluoride when reacts with calcium fluoride as shown below:
CaF2 + H2SO4 → CaSO4 + 2HF
It forms carbon when reacts with glucose, sugar, and starch.
C12H22O11 + (H2SO4) → 12C + 11 H2O
Uses of Sulphuric Acid
Sulphuric acid has lots of uses, some of which are as follows:
- It is used to produce various organic chemicals such as organic acids such as nitric acid, hydrochloric acid, and more. It is also required in the formation of some strong alkylating agents such as dimethyl sulphate. Further, ester and diesters are also derived from sulphuric acid.
- It is required for the manufacturing of synthetic detergents such as LABSA (Linear Alkyl Benzene Sulphonic Acid) and also for the formation of acid slurry (Dodecyl Benzene Sulphonic Acid).
- It is used to produce various dyes and pigments in coloring industry.
- It is also required to produce explosives. The explosive TATP (Triacetone triperoxide) is prepared from sulphuric acid, acetone and hydrogen peroxide.
- In the pharma industry, it is used as a solvent to synthesize a wide range of chemicals that also includes active pharmaceutical ingredients. For example, the alkylating agents that are used in chemotherapy are formed by using sulphuric acid.
- It is used in the oil refining process wherein sulphuric acid is used as a catalyst.
- It is also required in the metal processing process called pickling. In this process, impurities like rust or scale are removed from the surface of metals.
- It is also involved in the manufacturing of Rayon. The cellulose fibers are dissolved in a solution of Tetra Amine Copper (II) to form a blue liquid, which is injected into sulphuric acid to produce Rayon fibers.
- The manufacturing of lead-acid batteries also requires sulphuric acid. In these batteries, sulphuric acid is used as an electrolyte that allows the flow of current between the plates of the battery.
- It also helps in the harvesting of potatoes. A solution of sulphuric acid is sprayed over potato tops. It blackens the green tops within a few days, so the stems dry out and prevent them from tangling in the harvesting machine.
- Piranha solution is also prepared by using sulphuric acid. It is a strong oxidizing agent, which is generally used for cleaning mainly in the microelectronics industry.
Preparation of Sulphuric Acid
There are mainly two methods for the industrial preparation of sulphuric acid, which are as follow:
1) Contact Process:
This method comprises three steps as described below:
Step 1): Production of Sulphur Dioxide
The starting material required for the preparation of sulphuric acid is clean and dry sulfur dioxide (SO2) gas. It is produced by heating Sulphur or sulphide ores. The chemical reaction is as follows:
S (Sulphur) + O2 + Δ (Heating) → SO2
Or
4FeS (Iron pyrites or sulphide ore) + 7O2 + Δ (Heating) → 2Fe2O3 (Ferric Oxide) + 4SO2
Step 2): Formation of Sulphur Trioxide
In this step, the SO2 undergoes oxidation using V2O5 as a catalyst and in the presence of atmospheric oxygen to form Sulphur trioxide. This reaction is irreversible and takes place at a temperature of 400 to 450 degrees Celsius as shown below:
2SO2 + O2 + V2O5 ? SO3
Step 3) Absorption of Sulphur Trioxide into Sulphuric Acid
Sulphur trioxide cannot be dissolved in water directly as it forms fog. So, in this step, Sulphur trioxide is absorbed in around 98% H2SO4, which leads to the formation of oleum or fuming sulphuric acid, as shown below:
SO2 + H2SO4 → H2S2O7 (Oleum)
Step 4) Dilution of oleum
In this step, oleum is diluted with water to form sulphuric acid of the desired concentration.
2H2S4O7 + H2O →H2SO4
2. Lead Chamber Process
It is one of the most common methods for the preparation of sulphuric acid. This method uses wet SO2 in the presence of nitrogenous oxides. SO2 is oxidized with atmospheric oxygen to form Sulphur trioxide as shown below:
2SO2 + O2 → 2SO3
Afterwards, Sulphur trioxide is reacted with water to form H2SO4 as shown below:
SO3 + H2O → H2SO4
Conditions to get maximum yield of Sulphur trioxide
The main step in the preparation of sulphuric acid is the oxidation of SO2 to SO3. The conditions for maximum production of Sulphur trioxide as per Le Chatelier's principle are as follows:
- Low temperature: Low temperature promotes the oxidation of Sulphur dioxide as the forward reaction is exothermic. However, it should not be less than the optimum temperature of 720 K.
- High pressure: It also helps increase the yield of SO3. This is because the volume of the gaseous products is less than the volume of gaseous reactants. But, it should not be too much high as it may corrode the vessel used for oxidation. Pressure from 2 to 3 bar is appropriate.
- Use of catalyst: V2O5 vanadium pentoxide is used as a catalyst to increase the yield of SO3.
- Purity of gases: The gases can be purified before their oxidation in the presence of a catalyst.
| 
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now










