Tartaric AcidTartaric Acid is an alpha-hydroxy carboxylic acid. Its chemical formula is C4H6O6. It is an organic acid and is naturally found in various vegetables and fruits such as grapes, bananas, tamarinds, etc. It is also a by-product of winemaking. 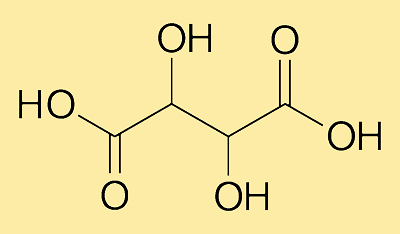
It also goes by the names 2, 3-dihydroxysuccinic acid, 2, 3-dihydroxybutanedioic acid, and racemic acid. It is a diprotic acid with a characteristic sharp tart taste. Its molecular weight or molar mass is 150.087 g/mol. Further, it is a dihydroxy derivative of dicarboxylic acid and its salts are called tartrates. Origin of Tartaric AcidIt is a natural acid, which is commonly found in nature as 2 stereoisomers. For the first time, it was isolated from potassium tartrate by Jabir lbn Hayyan in Iraq in the 7th century. Its chirality was discovered by Jean Baptiste Biot who found that it could rotate polarized light. Later, Louis Pasteur studied the shapes of tartaric acid crystals, which he found asymmetric. Commercial Production of Tartaric AcidIt can be produced by both natural and synthetic methods. In the natural method, the reddish precipitated salt from argol (sediment in wine vats) is used. So, it is produced commercially from the waste products left behind during making of wine. In synthetic way, it is prepared from maleic anhydride. Characteristics of Tartaric AcidIt is available in the form of colorless to white translucent fine crystals or granular crystalline powder. It is a solid material at room temperature owing to its high melting point: 170 degrees Celsius. And, its boiling point is 275 degrees Celsius. It is expensive than many acidulants such as citric acid and malic acid. All types of tartaric acid are soluble in water. When it reacts with bases, it forms salts. For example, cream of tartar (potassium hydrogen tartrate) and Rochelle salt (potassium sodium tartrate). When dissolved in water, it forms insoluble calcium tartrate precipitates. Tartaric Acid StructureIts molecule has 4 carbon atoms, 6 hydrogen atoms, and 6 oxygen atoms. It contains two hydroxy (OH) groups along with two carboxylic acid (COOH) groups. Further, it is a chiral molecule, which shows stereoisomerism: D-tartaric acid, L-tartaric acid, and meso-tartaric acid. Properties of Tartaric Acid
Uses of Tartaric Acid
Health HazardsIf it is inhaled, it is absorbed into the body and causes burning sensation and difficulty in breathing. Further, it can cause redness and irritation when comes in contact with skin and can also cause pain in the eyes. It is a muscle toxin, which prevents the production of malic acid and may cause death or paralysis in high doses. However, in low doses in food products, it is safe for human consumption.
Next TopicCarboxylic Acid
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










