VaporThe vapor or vapour is a substance present in the gaseous state at a specific temperature. We can convert it into a liquid by increasing the pressure without changing the temperature. Let's discuss how? The atoms of the gases are very loosely packed with large spaces between them. When the pressure is applied to a gas, the atoms are forced to come closer. It results in the change of the state of a substance from gas to liquid. Under the specified set of conditions, the substance in the vapor form can also exist in the liquid form. For example, Water The water in the form of vapor is known as steam, and ice in the form of solid. At very low temperatures, the water converts in the form of ice. After the water has reached its boiling point, it starts converting into steam. We have learned about two terms (condensation and sublimation) related to the states of the matter (solid, liquid, and gas). Let's discuss the relation of thee two terms with the vapor. CondensationCondensation refers to the change in the state of a substance from the gas to the liquid. It is the reverse of the vaporization state, which converts the state of matter from liquid to gas. In terms of vapor, we can say that the change of vapors into the liquid is known as condensation. 
The vapors can be converted into liquid in two ways:
Let's discuss a practical example of the condensation of vapors, which can be seen in the atmosphere. The warm air from the land rises upwards towards the atmosphere. At a point, it cools and loses its ability to hold the water vapors (gaseous state of the water). The excess water vapors in the atmosphere results condense to form the water droplets. The water droplets combine with the dust and smoke in the air form small cloud droplets. These cloud droplets are created again and again in a similar way. When it is present in large amounts, it combines and forms clouds. The clouds are the form of water present in the atmosphere. When the cloud becomes heavy enough with the water, it starts to fall on the Earth in the form of rain. The evaporation rate and condensation rate of liquids is shown below: 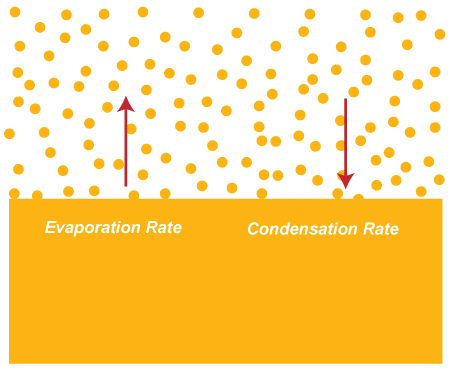
SublimationSublimation is defined as the state of transition from the solid to the gas without passing through the state of the liquid. The sublimation occurs at a specific temperature and pressure applied to the solid. The practical example of sublimation is moth balls, which directly gets converted from the solid to the gas. We can easily detect the smell of such balls and the reduction of their size as time passes by. 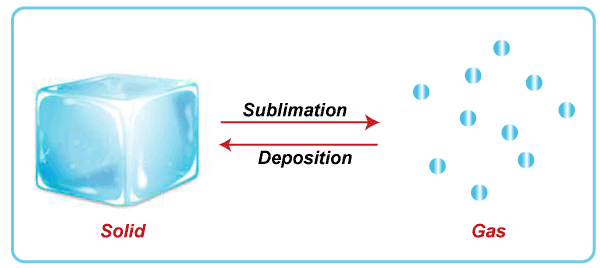
Properties of vaporThe properties of vapor are listed as follows:
Vapor pressureThe vapor pressure is defined as the equilibrium pressure exerted by the solid or liquid at a specific temperature. It does not depend on the contact of the vapors with the solid or liquid. The vapor pressure can be determined by the type of molecules that constituent a particular solid or liquid. It increases with the rise in temperature. We can also say that the pressure exerted by the vapor present over the surface of a substance is known as vapor pressure. According to the Raoult's of physical chemistry, the partial pressure of a component is defined as the product of the vapor pressure of the pure component and its mole fraction in the mixture. The partial pressure of the gas helps us to determine the amount of vapors present in a substance. Vapor vs. gasThe two terms vapor and gas are often confused. Though vapor is the gaseous state of a substance, it is a combination of the gaseous and liquid states in ordinary conditions. The gas is a single-state substance only, and it is not a mixture of any other state. There is no such physical or chemical difference between vapor and gas. The two terms are quite similar. But, in ordinary conditions, it may differ. Vaporization vs. EvaporationVaporization is defined as the gaseous state of a substance when the vapors are formed during its boiling process. It can also convert all the water into gas. Evaporation is also a type of vaporization, but it occurs below the boiling point of a substance. Examples of vaporsHere, we will discuss the most common examples of vapors that can be seen around us in our daily life. The examples are listed as follows: 
Next TopicKirchhoff's law
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










