What are the Advantages and Disadvantages of Electroplating?A common method for improving and polishing metals that are utilized across many different sectors and for a variety of purposes is electroplating. Although electroplating is widely used, relatively few people outside of the business are aware of the procedure, what it is, and how it operates. You need to understand how electroplating functions as well as your alternatives for material and procedure if you're thinking about using it in your next production process. Electroplating: What is it?Electrodeposition is another name for electroplating. The procedure includes depositing materials using an electric current, as the name would imply. The substrate, a workpiece, is coated with a thin coating of metal as a consequence of this procedure. The main purpose of electroplating is to modify an object's physical characteristics. Along with increasing thickness, this procedure can boost an object's wear resistance, corrosion protection, or aesthetic appeal. 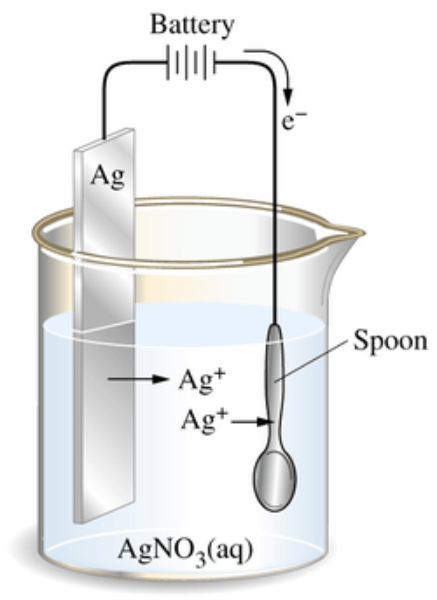
Although electroplating appears to be a cutting-edge technique, it has been practiced for many years. One of the earliest electroplating experiments took place in the early eighteenth century, and in the first part of the 19th century, Brugnatelli codified the procedure. Following Brugnatelli's research, the electroplating technique spread throughout Europe. The electroplating method changed during the following two centuries to keep up with demand as manufacturing techniques expanded during the industrial revolution and two world wars, giving rise to the procedure that the Sharretts Plating Company uses today. Electroplating ProcedureAn electric current is used in the electroplating process to break down the metal and deposit it on a surface. The four main parts of the procedure are as follows:
The power supply delivers a direct current (DC) to the anode once the anode and cathode have been inserted into the solution and connected. The metal oxidizes as a result of this current, permitting metal atoms to dissolve as positive ions in the electrolyte solution. The metal ions are subsequently moved by the current to the negatively charged substrates and deposit a small coating of metal onto the object. Take the use of gold plating on metal jewelry as an example. The anode of the circuit is gold-plated metal, and the cathode is the metal jewelry. Both are dissolved in solution, and the gold is given DC electricity to dissolve it. The gold atoms that have been dissolved then stick to the jewelry's base metal surface to form a gold coating. These elements include the following:
What Metals are Utilized in the Process of Electroplating?Individual metals or different alloy combinations (which can add value to the electroplating process) can be plated. The following metals are some of the most often used for electroplating: Copper: Due to its heat resistance and conductivity resistance, copper is frequently utilized. It is frequently employed to strengthen the bond between materials. Zinc: Zinc is very resistant to corrosion. To improve this quality, zinc is frequently alloyed with other metals. Zinc, for instance, is highly resistant to air corrosion when alloyed with nickel. Tin: This shiny, matte metal is very solderable, corrosion-resistant, and safe for the environment. Additionally, it is less costly than other metals. Nickel: Heat treatment can increase nickel's already high wear resistance. Because they provide elemental resistance, hardness, and conductivity, their alloys are also extremely important. Additionally prized are electroless nickel plating's hardness, magnetism, reduced friction, and corrosion resistance. Gold: This priceless metal is highly conductive and aesthetically pleasing, with high corrosion, tarnish, and wear resistance. 
Silver: Silver is extremely malleable and ductile. It has remarkable resistance to contact wear and has outstanding aesthetic value. However, it is not as weathering resistant as gold. In situations where both electrical and thermal conductivity is required, it also serves as a substitute for gold. Palladium: Due to its hardness, resistance to corrosion, and lovely polish, this brilliant metal is frequently employed in place of gold or platinum. This metal acquires outstanding rigidity and plating quality when alloyed with nickel. The most suitable electroplating substance for your application depends on several parameters, including cost, substrate composition, and desired results. Several Plating MethodsThere are several plating methods available, and each one has a variety of uses. Below are some of these electroplating kinds in further detail: 1. Barrel platingLarge groupings of tiny components can be plated using the barrel plating technique. In this procedure, components are put within a barrel that has an electrolyte solution inside of it. The barrel is spun while the electroplating process is taking place, agitating the pieces to provide consistently equal finishes. Barrel plating is an inexpensive, effective, and adaptable option that works well on tiny, robust items. 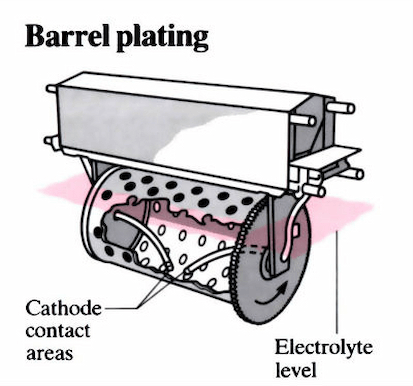
2. Rack electroplatingWhen electroplating large groupings of components, rack plating, also known as wire plating, is a viable choice. This technique puts each component in immediate communication with the power supply by arranging it on a wire rack. This choice is ideal for more sensitive items that really can undergo barrel plating, albeit being more costly. It is crucial to remember that rack plating is much more challenging for objects that are electrically conductive or have irregular shapes. 3. Electroless platingElectroless plating also referred to as autocatalytic plating, is a method similar to electroplating but does not subject the component to electricity. Instead, a chemical process is used instead of an electric one to dissolve and deposit the plating metal. Despite being beneficial for parts that cannot be operated by electrical currents, this approach is more expensive and less effective than others. Although these techniques achieve electrodeposition in various ways, they all share the same fundamental ideas. Electroplating ApplicationsWhile electroplating is frequently employed in a variety of sectors to enhance the visual appeal of a base material, it also serves other functions. Among them are the following:
Electroplating-User Related IndustriesElectroplating provides options for businesses seeking corrosion protection, better durability, or improved electrical conductivity. Because of this, electroplating is often employed in many different sectors. Below are a few of the sectors that SPC supports and how they use electroplating.
Electroplating is used in several different areas, including the military, defense, and gun sectors. Electroplating is preferred by all of these sectors because of its practical qualities, cheap cost, and adaptability of use. Example of ElectroplatingApplications for electroplating may be found in several distinct sectors and fields. Here are a few of these in more detail:
Advantages of ElectroplatingThere are several advantages to electroplating for components. The following are some unique advantages of electroplating:
Some advantages provided are unique to metals. For instance, nickel plating can aid to lower friction, which lowers wear & tear and increases the longevity of the item. On the other hand, sharp protrusions during production that might cause component damage are avoided by using zinc-nickel alloys. In many applications, copper is also employed expressly as an undercoating because it makes it easier for other metal coatings to adhere to, improving the finished product's top quality. Disadvantages of ElectroplatingThe many benefits of electroplating have been examined, but it would only be fair to consider the drawbacks of this crucial process as well. Let's look at the drawbacks of electroplating:
Next TopicGibbs Energy
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share












