Difference Between Molarity and MolalityWhat is Molarity?The exact number of moles of a substance or the solute that is dissolved in 1 litre of a solution (solute and solvent combined) is known as its molarity. Therefore, the following is the molarity calculation formula: Molarity (M) = moles of solute / Volume of solution in Liters 
Molarity is also known as molar concentration. Consequently, a molar concentration measurement is based on the amount of liquid, a substance is dissolved in. It is crucial to understand that the volume is in litres, so if your volume, for example, is in millilitres, you might need to convert it first. A given amount of solute is added to a volumetric flask to create a molar concentration, which is then filled with liquid until it reaches the 1-litre level. For instance, sugar can be concentrated to a particular molar ratio. Prior to adding water until 1 litre is attained, the sugar's weight must be converted to moles. To determine molarity, you require the solute's molecular weight, but in most cases, the solute will be given weight. Therefore, you must first convert grams to moles. This can be accomplished by utilising the periodic table to determine the solute's molar mass. The molar concentration formula can be altered to solve for both volume and moles. Temperature and pressure variations have an impact on volume. For instance, as the temperature rose, the volume would as well. This implies that where there are temperature variations, there will be some doubt about accuracy. The liquid may constrict if the temperature drops low enough, which would result in a smaller volume of a solution but the same number of moles. A sufficiently high temperature, however, may cause the liquid to expand, which would result in more solution being present even though there would still be the same number of moles. This would cause the molarity to decrease. After a chemical has been diluted, its concentration can be determined using molarity. When exact precision is not necessary, molarity can be employed. However, because it is a volumetric measurement, it is affected by variations in temperature. Therefore in some circumstances, it would not be appropriate to use. In some circumstances, molarity and molality can be synonymous; for example, 1 kg of water weighs 1 litre. What is Molality?
The number of moles of a substance or the solute, that is present in 1 kilogram of solvent, is known as molality. To determine molality, use the following equation: m = moles of solute/ mass of solvent in kg 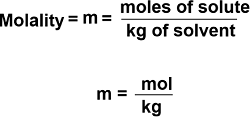
The terms molality and molal concentration are interchangeable. Weighing out a specific quantity of sugar is one way to create a molal concentration. The molecular mass of the sugar must then be used to convert this into the number of moles. After that, water is added to a beaker until it weighs 1 kg and the beaker is weighed again. The water in the beaker is then added, and the sugar is subsequently dissolved. Since molality is determined by mass rather than volume, it has the advantage over molarity in that it is unaffected by variations in temperature and pressure. Since the solvent's mass is not affected by temperature in the same way that a substance's volume is, molality rather than molarity provides a more precise method for estimating concentration. Given that 1 litre of water weighs 1 kg, it is possible that the molarity and molality of water are the same. However, this might not apply to all liquids. This means that when considering colligative properties, molality must be employed. Molality is more precise and offers more concentration precision, but it requires more time to prepare because the solute must be added to the weight of a solvent. Liquid solvents must be weighed if they are to be used. A gravimetric apparatus and an analytical balance can be used to weigh the solvent in this process. Difference between Molarity and Molality:
Question ExamplesQues 1. Write down the connection between molarity and molality. Answer: The molarity of a solution is the sum of all the moles of solute in one litre of a solution. It is dependent on a few variables, including volume, temperature, and pressure. M designates it. Another name for it is molar concentration. The number of moles of a solute per kilogram of solvent is used to determine the molality of a solution. It is based on the solvent's mass. M designates it. Another name for it is Molal Concentration. Molarity and Molality are related by the following formula: m = (1000 M)/ [1000d - (M X Mb)] M= (m x 1000 x d)/ 1000+ (m x Mb) where, m = molality, M = molarity, and d = solvent density. The solution's molecular mass is denoted by Mb. Question 2. If the molality of a solution is 1.45 mol/kg and a solvent density of 0.15 kg/m3, determine its molarity. Answer: The value of molality m is 1.45 mol/kg, Let the mass of the solution be 1 kg, D = 0.15 kg/m3 M= (m x 1000 x d)/ 1000+ (m x Mb) M= (1.45 X 1000 X 0.15)/ 1000+ 1.45 X 1 M= 0.2175 mol/L As a result, the solution has a molarity of 0.2175 mol/L. Question 3: When 180 g of urea is dissolved in 1200 g of water, a solution with a density of 1.25 g/mL is created. Determine the solution's molarity. (The molecular weight of urea is 60 g/mol.) Answer: In light of this, the urea's molecular mass is 60 g/mol. Given the weight of urea = 180 g Number of urea moles = 180/60 = 3 moles The volume of the solution is equal to its mass times its density. The solution's mass is 1200+180gm, or 1380gm. Volume = 1104mL = 1.104L (1380gm/1.25gm/mL) The formula for urea molarity is M =no. of moles/volume in L M = 3 /1.104 = 2.72 mol/L. The molarity of the solution is 2.72 mol/L. Question 4. A solution with a density of 1.12 g/ml was produced by dissolving 120g of urea in 1000g of water. The solution's molarity is? Answer: The weight of urea is 120 g, water is 1000 g, and the density is 1.12 g/ml. Knowing that density equals mass/density Mass / Density = volume = (120+1000)/ 1.12 = 1120/1.12 = 1000 ml = 1 litre. Molarity = Number of Mole Solutes/Volume in Litre Weight/Molecular Weight = Number of Mole = 120/60 Moles Number = 2 Moles Molarity = 2/1 = 2 M Question 5. Determine the molality of 15.00 M HCl with a density of 1.0745 g/cm3 in question four. Answer: 1) Assume there are 1000 millilitres of solution on hand. This litre of a 15-molar solution contains: (1.000 L) x (15.00 mol/L) = 15.00 moles of HCl 546.9135 g of HCl are produced from 15.00 mol times 36.4609 g/mol. 2) Calculate the mass of the solvent using the density (1.0745 g/cm3) and volume of solution (1000ml): 1074.5 g of solution - 546.9135 g of HCL = 527.5865 g or 0.5275865 kg. 3) Determine the molality: 15.00 mol/0.5275865 kg = 28.43 m. A Few Things to Consider
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










