Difference between Molecularity and Order of ReactionIn the field of chemistry or chemical kinetics, we often hear two terms, namely "molecularity" and "order of reaction". Both these terms have specific roles and significance in their respective fields. Furthermore, they are often used interchangeably by many people. However, they are not the same and possess various distinctive differences from each other. In this article, we highlight the notable differences between these two important terms of chemistry. What is Molecularity?Molecularity is a concept in chemistry that refers to the numerous molecules which take part in a targeted chemical reaction. In other words, it measures how many reactants come together to form the products of the reaction. The term "molecularity" is often used to describe elementary reactions, which are simple reactions that occur in a single step without any intermediate products or steps. In this section, we explore the properties of molecularity and how it affects chemical reactions. Molecularity refers to the number of reacting molecules, such as atoms, that participate in a chemical reaction. It is a crucial concept in chemical kinetics that helps understand the rate of a reaction. The molecularity of a chemical reaction can be easily calculated by examining the stoichiometry of that particular reaction. Three different types of molecularities exist, such as unimolecular, bimolecular, and termolecular.
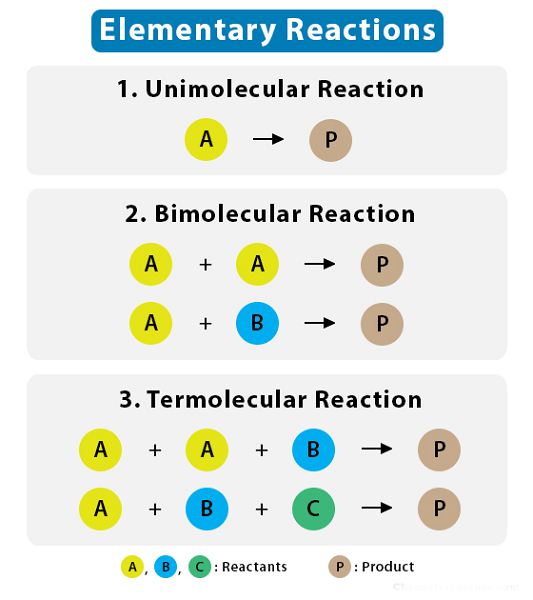
Molecularity and Reaction RateThe molecularity of a chemical reaction can make a huge difference in the rate of the reaction. Generally, the higher the molecularity of a reaction, the slower the reaction rate. This is because reactions with a higher molecularity require more molecules to come together and collide with enough energy to initiate the reaction. For example, a reaction with a molecularity of two (a bimolecular reaction) requires two molecules to collide. In contrast, a reaction with a molecularity of three (a termolecular reaction) requires three molecules to collide. Since multiple molecules are less likely to collide simultaneously, termolecular reactions are typically much slower than bimolecular reactions. Molecularity and Reaction MechanismsThe molecularity of a reaction also plays a crucial role in determining the reaction mechanism or the series of steps that occur during a reaction. Elementary reactions, which have a molecularity of one, typically occur in a single step and do not have an intermediate product. In contrast, complex reactions with higher molecularity often involve multiple steps and intermediate products. For example, a reaction with a molecularity of two could occur in two steps, with an intermediate product formed during the first step before being consumed in the second step. Molecularity and Reaction KineticsMolecularity is also an essential factor in reaction kinetics or the study of the rate at which reactions occur. For example, a reaction with a molecularity of two may have a rate that depends on the concentration of both reactants, making it a second-order reaction. In contrast, a reaction with a molecularity of three may have a rate that depends on the concentration of three reactants, making it a third-order reaction. Molecularity and Reaction EquationsThe molecularity of a reaction can also be expressed in the chemical equation for the reaction. For example, a reaction with molecularity of one can be represented by the following equation: A ? B In this case, a single molecule of reactant A is transformed into a single molecule of product B in a single step. In contrast, a reaction with a molecularity of two can be represented by the following equation: A + B ? C In this case, two reactants A and B molecules form a single product, molecule C. Finally, the following equation can represent a reaction with a molecularity of three: A + B + C ? D In this case, three reactants A, B, and C, molecules come together to form a single product, molecule D. What is the Order of Reaction?Chemical reactions occur in nature and are crucial to various industrial and biological processes. However, not all chemical reactions occur at the same rate or require the same energy input. One of the critical concepts in chemical kinetics is the order of the reaction. This concept interlinks the reactants' concentration and the reaction rate at huge rates. The order of reaction can be called the power to which the concentration of the reactants is raised in the rate equation for a chemical reaction. The order of the reaction is determined by calculating the reaction rate as the concentration of one or more reactants is changed while keeping all other factors constant. Firstly, zero-order reactions have a constant rate independent of the reactant's concentration, which implies that the reaction rate is unaffected by variations in the reactant's concentration. A common example of a zero-order reaction is the decomposition of hydrogen peroxide in the presence of a catalyst. The rate of breakdown in this process is unaffected by hydrogen peroxide concentration. Rate=k, where k is the rate constant, is the rate law for a reaction with zero order. Secondly, first-order reactions have a rate proportional to one reactant's concentration. This further asserts that the reaction rate goes up as the reactant's concentration increases. The decay of radioactive isotopes is one of the finest examples of a first-order reaction. In this reaction, the decay rate is proportional to the concentration of the radioactive isotope. Rate=k[A], where [A] is the concentration of the reactant and k is the rate constant, is the formula for a first-order reaction. Thirdly, the rate of reaction in second-order reactions is either proportional to the square of the concentration of one reactant or the product of the concentrations of two reactants. This implies that the reaction rate rises as the concentration of the reactants rises. The conversion of two iodine molecules into one iodine monochloride molecule is a typical illustration of a second-order process. The reaction rate of such reactions increases as the product of the reactant concentrations increases in density. The rate law for a second-order reaction is rate=k[A]^2 or rate=k[A][B], where k is the rate constant and [A] and [B] are the concentrations of the reactants. 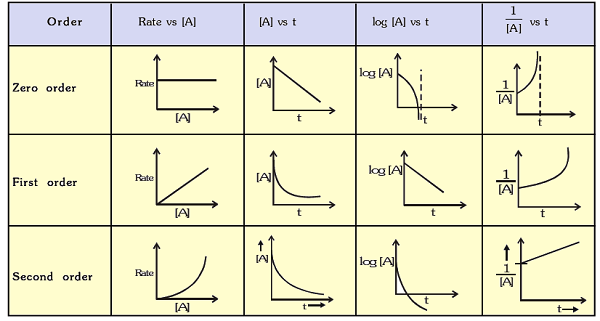
Finally, the rate of higher-order reactions is inversely correlated with the concentration of three or more reactants. These responses are less frequent and frequently have intricate mechanics. The conversion of carbon monoxide and nitrogen dioxide into nitrogen and carbon dioxide is a typical illustration of a higher-order process. The product of the concentrations of the three reactants determines the reaction rate in this process. Rate=k[A]m[B]n[C]p, where k is the rate constant and m, n, and p are the orders of the reactants, is the rate law for a higher-order reaction. The Properties of Order of Reaction
To contemplate the order of reaction experimentally and logically, several methods can be used. The most common methods are the initial rate method, the method of half-lives, and the graphical method. The initial rate method involves measuring the rate of the reaction at different concentrations of the reactants and determining how the rate changes concerning changes in the concentration of each reactant. The method of half-lives involves measuring the time it takes for the concentration of the reactants to decrease by half and plotting the data in a graph to determine the order of reaction. The graphical method involves plotting the concentration of the reactants versus time and determining the reaction order based on the curve's shape. Molecularity Vs. Order of ReactionThe term "molecularity" describes the quantity or number of molecules or particles engaged in the step that determines the rate of a chemical reaction. Simply put, this implies that it is the quantity or number of reactant particles that collide to produce the products in a single step. Elementary reactions, which are chemical processes that take place in a single step, are described by the theoretical idea of molecularity. The quantity of molecules or particles involved in an elementary process determines its molecularity. An example of a bimolecular or second-order reaction is one in which two molecules collide. Similar to this, a reaction that involves three molecules colliding is referred to as trimolecular or third-order. On the other hand, the order of reaction refers to the experimentally determined relationship between the concentration of reactants and the reaction rate. The order of reaction can be zero, first, second, or even fractional. Experiments that vary the concentration of one reactant while maintaining the concentration of the other reactants constant are used to determine the sequence or order of the reaction. The rate equation's definition of the order of reaction for a given reactant is the degree to which its concentration is increased. For instance, the order of a reaction involving reactant A is two or second-order if the reaction rate is proportional to the square of the reactant's concentration. 
One of the main differences is that molecularity is a theoretical concept used to describe elementary reactions. In contrast, the order of the reaction is an experimentally determined quantity that applies to any type of chemical reaction, including complex reactions that occur in multiple steps. Another difference is that molecularity is always an integer, whereas the order of reaction can be a fraction or a decimal. Molecularity is also independent of the reactants' concentration, whereas the reaction order is dependent on the concentration of the reactants. Furthermore, molecularity can be used to predict the rate law for an elementary reaction, whereas the order of reaction cannot be used to predict the mechanism of a reaction. The rate law for an elementary reaction is determined solely by the molecularity of the reaction. The product of the concentrations of the two reactants, for instance, determines the rate law for a bimolecular reaction. Contrarily, the rate law for a trimolecular reaction is proportional to the sum of the concentrations of the three reactants. The order of a reaction, on the other hand, says nothing about how a reaction works. Difference between Molecularity and Order of Reaction
ConclusionWhile molecularity and order of reaction are important concepts in chemical kinetics, they refer to different aspects of the rate of a chemical reaction. Molecularity is related to the reaction mechanism and the rate-determining step. Contrarily, the order of the reaction is established experimentally and is based on the correlation between reactant concentration and reaction rate. Understanding the differences between these two concepts is important for understanding the kinetics of chemical reactions and predicting chemical systems' behavior.
Next TopicDifference between
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










