Advantages and Disadvantages of Paper ChromatographyThe invention of paper chromatography by Martin and Synge in 1943 was the first method for surveying plant constituents and their separation and identification. Paper chromatography is a technique for separating colored chemicals or substances. It is now employed as a teaching aid because other chromatography techniques, such as thin-layer chromatography, have mostly been supplanted in the lab. There are three components to the setup. Two solvents are employed in two-dimensional paper chromatography, which rotates the paper 90 degrees in between. This helps separate combinations of substances with comparable polarities, such as amino acids. 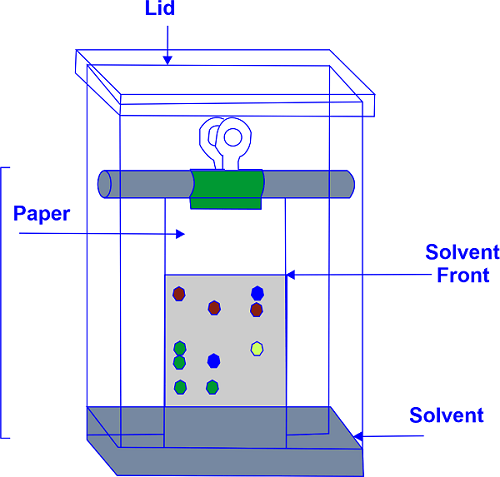
In contrast to paper chromatography, TLC uses an adsorbent layer as its stationary phase (often silica gel or aluminium oxide). In difference, the less absorbent paper serves as the stationary phase in paper chromatography. Pigments and Polarity in Paper chromatographyPaper chromatography is one method for evaluating compound purity and identifying substances. Paper chromatography is a helpful tool because it is quick and uses small amounts of material. Separations using paper chromatography employ the partition principle. Substances are transported among a stationary phase as well as a mobile phase in paper chromatography. The stationary phase is water that has become wedged between the paper's natural fiber. The samples advance the stationary phase, and the mobile phase enhances it.The components will easily separate depending on how firmly the sample's components diffuse onto the stationary phase compared to how quickly they dissolve in the mobile phase. 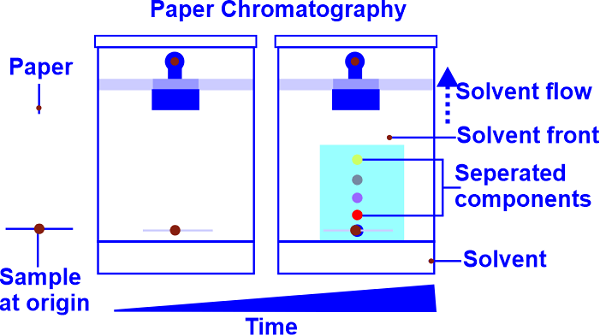
When filter paper with a colored chemical sample is submerged in a solvent at one end, the colors separate from the sample. The compounds in the mixture separate due to differences in their affiliations in both stationary and mobile phase solvents caused by the capillary action of a paper's pores. The sample must contain many types of molecules if it has multiple colors. Because each type of molecule has a unique chemical structure, there is a likelihood that each one will have at least a slight variation in polarity, giving each one a unique solubility in the solvent. Due to their varying solubilities, the various color molecules dissolve at different locations as the solvent goes up the paper. A molecule is more soluble the higher it migrates up the paper. Extremely non-polar substances will not dissolve in highly polar solvents. This holds for both a very polar chemical and a very non-polar solvent. It is crucial to realize that the more polar the color is, the higher it will rise on the paper when using water (a highly polar solvent). What Does Paper Chromatography Do?
Principle of Paper ChromatographyThe paper chromatography principle involved partition or adsorption chromatography. Due to the separation or distribution of the components between liquid phases, partition chromatography is involved. A mobile phase travels through the filter paper, which hosts water in its pores. The mixture dissociates when the mobile phase moves. The compounds in the combination separate due to differences in their affinities in stationary and mobile phase solvents caused by the capillary action of the pores in the paper as in adsorption chromatography where the liquid phase is the mobile phase and the paper's solid surface acts as the stationary phase. Procedure for Paper Chromatography
Because it is straightforward, ascending type or radially paper chromatography is frequently utilized. It is also simple to use, produces a faster chromatogram, and takes less time to complete.
The filter paper is chosen based on the pores' size and the sample's quality.
Preparing a sample involves dissolving it in an appropriate solvent (inert to the substance being analyzed) that will be utilized to create the mobile phase.
Samples should be spotted in the proper position using a capillary tube. Chromatogram development is detected by dipping the paper in the mobile phase. The mobile phase moves over the sample on the paper because of the capillary action of the paper.
The paper is dried with an air drier after the chromatogram has been developed. Spatter the detecting solutions on the chromatogram-developed paper and let it dry to detect the sample chromatogram spots. Paper Chromatography Types
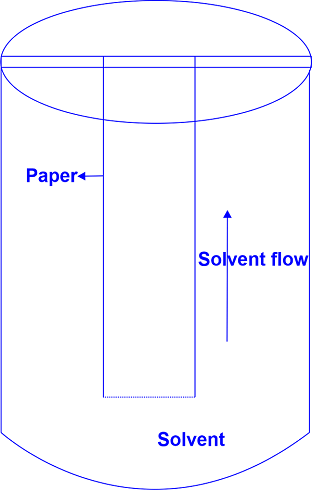
The solvent travels up the chromatographic paper at this point. Organic and inorganic compounds are separated using descending and ascending paper chromatography. The sample and solvent ascend.
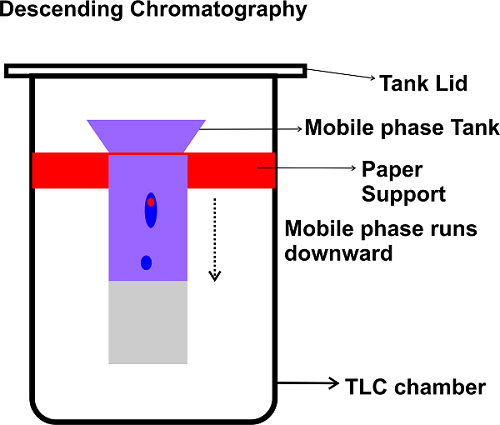
The chromatogram is developed by allowing the solvent to run down the paper. The mobile phase is positioned at the top of the solvent holder. The spot remains at the top, and the solvent flows from above.
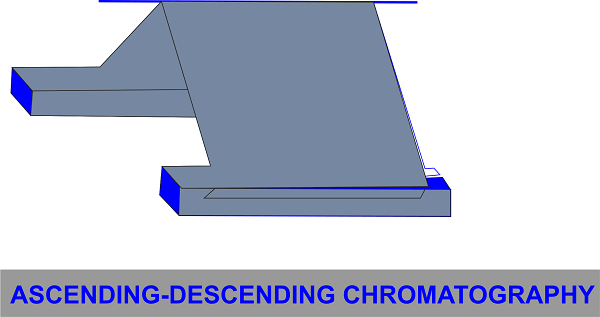
This combines the first two methods stated above. To allow the paper to descend after passing the rod, the ascending chromatography component can be wrapped over the rod.
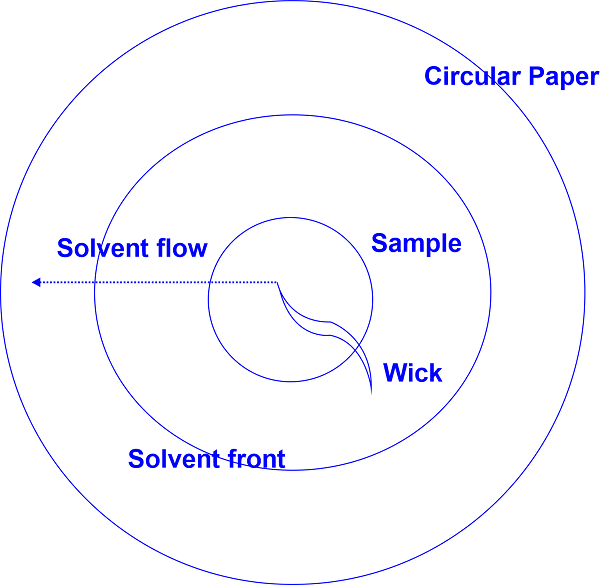
The sample is positioned in the middle of a circular filter paper. After the spot has dried, the filter paper is positioned horizontally on a liquid-filled Petri dish with the paper's wick submerged in the solvent. Through the wick, the solvent is released, dividing the constituents into concentric rings.
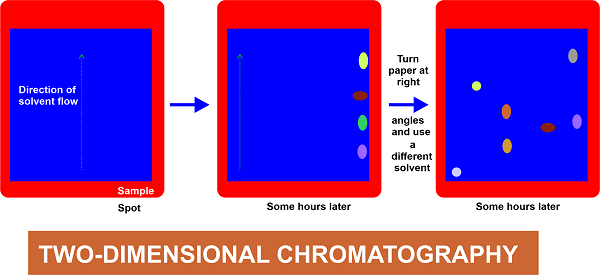
This method necessitates the use of a square or rectangular piece of paper. The samples are placed in one of the corners, and development is performed perpendicular to the first run's direction. Paper Chromatography's Standard Applications
A practical technique for removing colored pigments from a combination. On filter paper, a few drops of the colored pigment mixture are applied, and the paper is slowly submerged in the solvent. Based on the polarity of the molecules in the mixture, the solvent dissolves them as it lifts the paper. As the solvent crosses the filter paper, the molecules of each pigment depart from the solution at various locations due to their various polarity. Each pigment rises to a particular level on the chromatography paper, and the procedure separates them. Plant pigments can be separated using it.
The concentration of the reactants in a chemical reaction gradually drops as the concentration of the products increases. One can get a decent picture of how the reaction develops by identifying the reactants and generating the chromatogram over a range of periods. Densitometers allowed for quantitative estimations. However, the method was typically utilized for qualitative monitoring. Rapid spectroscopic techniques restrict paper chromatography as a reaction monitoring option.
Paper chromatography has been employed to isolate and purify the relevant compounds. The paper's differentiating elements are cut out, dissolved in the proper solvents, and their absorption at particular wavelengths is identified using spectro-photometric techniques.
Because paper chromatography can be done successfully with extremely few amounts of material, it is helpful in forensic science for examining crimes. This method is used to gather and examine samples from crime scenes. Used for DNA and RNA identification. Paper chromatography is another technique used in pathological laboratories to find compounds or alcohol in the blood.
Foods are coloured with both natural and artificial ingredients to increase their acceptability and popularity. The main application of paper chromatography has been for the examination of food colours in ice creams, sweets, drinks, jams, and jellies.
A complex combination of organic components can be identified or detected using paper chromatography, including specific organic compounds like amino acids and carbohydrates. It can be used to divide amino acids from anions. 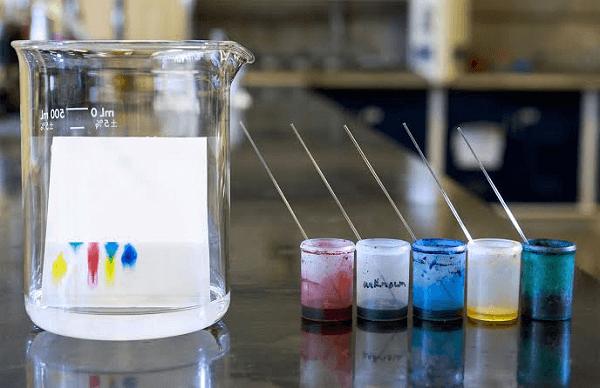
Advantages of Paper Chromatography
Disadvantages of Paper Chromatography
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share









