What is the full form of PSAPSA: Pressure Swing AdsorptionPSA stands for Pressure Swing Adsorption. It is a process that is used when there is a need to remove a single gas from a mixture of gases. With PSA, it becomes so much easier to separate the desired gas from the mixture. 
As we know, the air is a mixture of gases, and therefore the process of PSA is commonly used to separate different gases from the air. Air contains gases like Oxygen, Nitrogen, Argon, Carbon Dioxide and others. Gases like Oxygen and Nitrogen are used widely in many industries for different purposes. Therefore, the process of PSA helps in separating and collecting these gases so that they can be utilized for certain tasks by industries. The industries that use these gases the most are fabrication and cutting, health care, wastewater treatment, chemical manufacturing, and food packaging. Understanding each term of PSAPressureIn this process, the term "pressure" is used to describe the high pressure that must be between 5-10 bar to separate the gases from the mixture. SwingThe next word, i.e. "swing", shows that two different tanks are required in the process of separating the air. Both the tanks perform different functions; one of the tanks is pressurized, and the other tank is depressurized so that the air that is being compressed in the one tank can easily move to the other tank where it is depressurized, which makes the compressed air move from one tank to another or say swing from one tank to another in the process of separation. AdsorptionThe word adsorption here means that while separating gas from the mixture of gases, there should be something which adsorbs the other non-required gases so that the gas which we are separating from the mixture only gets out and not the other gases. For example, an adsorbent named Zeolite is used when oxygen is separated from the air. Zeolite is found naturally but can also be made artificially, and it contains compounds like Silicon and Aluminium. Zeolite has some pores due to which nitrogen from the air is absorbed into it when it is used as an adsorbent, separating the oxygen from the mixture. Working of the PSALet us understand the working of the PSA technology with the example of separating oxygen, a gas, from the air. 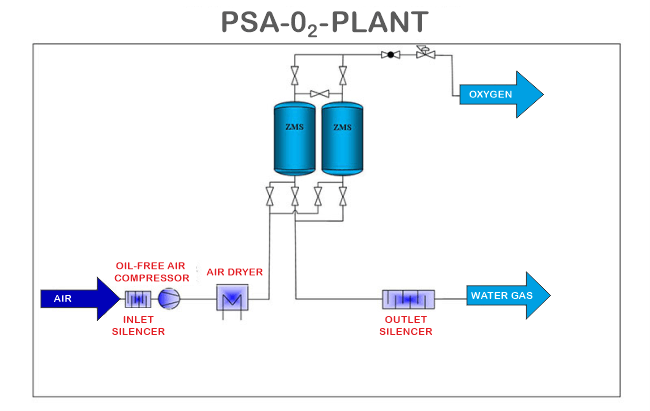
As we mentioned above that, to perform this process, two different tanks are required. Suppose we are separating oxygen, then the adsorbent that we use in this process will be Zeolite. The adsorbent, which is Zeolite here, in this case, is placed in the form of light pallets. After that, dry compressed air is directed into the first tank through a valve. In the first tank, the pressure is set manually, which usually stays around 5-10 bar. Additionally, as an adsorbent, Zeolite is placed in the tanks, which helps adsorb all the Nitrogen present in the supplied air. This nitrogen adsorption takes place when the pressure in the tank increases. All the adsorbed nitrogen gas is then released or stored in other vessels so that it can be used for other purposes. When the first tank gets full, and all the unwanted nitrogen settles down in the first tank, the oxygen separates from the first tank. The pressure of the first tank is then slowly reduced, all the compressed air is transferred to the second tank, and the waste which has been settled down is exhausted from the waste outlet. Then in the second tank, the compressed air is sent with the help of a valve. The inlet of the first tank is then closed, and all the compressed air is directed towards the second tank only. As the compressed air enters the second tank, the pressure in the second tank increases, and the same process of adsorption of nitrogen takes place in the second tank because of the presence of adsorbent, i.e. Zeolite. This cycle of adsorption, separation, the building of pressure and reducing pressure and the introduction of compressed air continues again accordingly. Due to the continuity of this cycle, there is a constant flow of oxygen separated from the tanks. We don't need to wait for the first vessel to complete and then another when the process is ongoing. Instead, the air is continuously directed, and oxygen is continuously received. This process of separation of gases is also economically viable and not high-priced.
Next TopicFull Form
|
 For Videos Join Our Youtube Channel: Join Now
For Videos Join Our Youtube Channel: Join Now
Feedback
- Send your Feedback to [email protected]
Help Others, Please Share










